Page 6 - che142 solutions ebook
P. 6
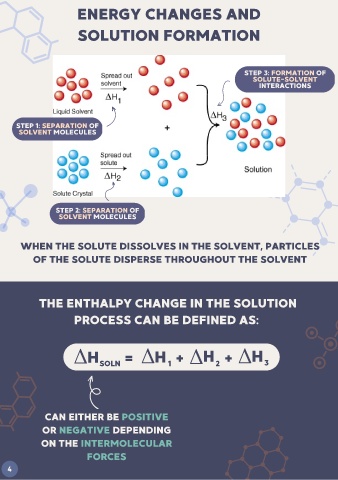
ENERGY CHANGES AND
SOLUTION FORMATION
STEP 3: FORMATION OF
SOLUTE-SOLVENT
INTERACTIONS
STEP 1: SEPARATION OF
SOLVENT MOLECULES
STEP 2: SEPARATION OF
SOLVENT MOLECULES
WHEN THE SOLUTE DISSOLVES IN THE SOLVENT, PARTICLES
OF THE SOLUTE DISPERSE THROUGHOUT THE SOLVENT
THE ENTHALPY CHANGE IN THE SOLUTION
PROCESS CAN BE DEFINED AS:
H SOLN = H 1 + H 2 + H 3
CAN EITHER BE POSITIVE
OR NEGATIVE DEPENDING
ON THE INTERMOLECULAR
FORCES
4