Page 9 - che142 solutions ebook
P. 9
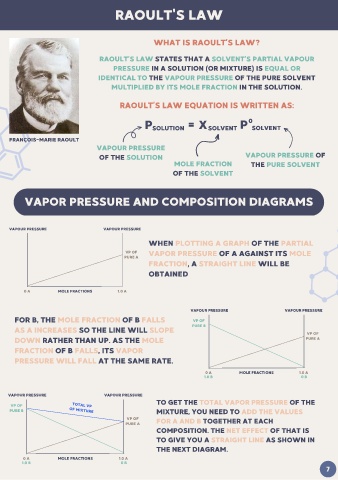
RAOULT'S LAW
WHAT IS RAOULT’S LAW?
RAOULT’S LAW STATES THAT A SOLVENT’S PARTIAL VAPOUR
PRESSURE IN A SOLUTION (OR MIXTURE) IS EQUAL OR
IDENTICAL TO THE VAPOUR PRESSURE OF THE PURE SOLVENT
MULTIPLIED BY ITS MOLE FRACTION IN THE SOLUTION.
RAOULT’S LAW EQUATION IS WRITTEN AS:
P SOLUTION = X SOLVENT P 0 SOLVENT
FRANÇOIS-MARIE RAOULT
VAPOUR PRESSURE
OF THE SOLUTION VAPOUR PRESSURE OF
MOLE FRACTION THE PURE SOLVENT
OF THE SOLVENT
VAPOR PRESSURE AND COMPOSITION DIAGRAMS
VAPOUR PRESSURE VAPOUR PRESSURE
WHEN PLOTTING A GRAPH OF THE PARTIAL
VP OF VAPOR PRESSURE OF A AGAINST ITS MOLE
PURE A
FRACTION, A STRAIGHT LINE WILL BE
OBTAINED
0 A MOLE FRACTIONS 1.0 A
VAPOUR PRESSURE VAPOUR PRESSURE
FOR B, THE MOLE FRACTION OF B FALLS VP OF
AS A INCREASES SO THE LINE WILL SLOPE PURE B
VP OF
DOWN RATHER THAN UP. AS THE MOLE PURE A
FRACTION OF B FALLS, ITS VAPOR
PRESSURE WILL FALL AT THE SAME RATE.
0 A MOLE FRACTIONS 1.0 A
1.0 B 0 B
VAPOUR PRESSURE VAPOUR PRESSURE
TO GET THE TOTAL VAPOR PRESSURE OF THE
VP OF TOTAL VP
PURE B OF MIXTURE MIXTURE, YOU NEED TO ADD THE VALUES
VP OF FOR A AND B TOGETHER AT EACH
PURE A
COMPOSITION. THE NET EFFECT OF THAT IS
TO GIVE YOU A STRAIGHT LINE AS SHOWN IN
THE NEXT DIAGRAM.
0 A MOLE FRACTIONS 1.0 A
1.0 B 0 B
7