Page 8 - che142 solutions ebook
P. 8
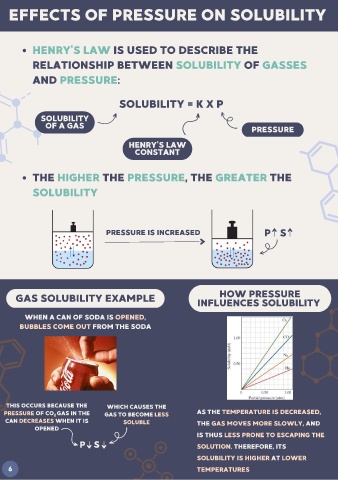
EFFECTS OF PRESSURE ON SOLUBILITY
HENRY'S LAW IS USED TO DESCRIBE THE
RELATIONSHIP BETWEEN SOLUBILITY OF GASSES
AND PRESSURE:
SOLUBILITY = K X P
SOLUBILITY
OF A GAS PRESSURE
HENRY'S LAW
CONSTANT
THE HIGHER THE PRESSURE, THE GREATER THE
SOLUBILITY
PRESSURE IS INCREASED P S
HOW PRESSURE
GAS SOLUBILITY EXAMPLE INFLUENCES SOLUBILITY
WHEN A CAN OF SODA IS OPENED,
BUBBLES COME OUT FROM THE SODA
THIS OCCURS BECAUSE THE WHICH CAUSES THE
PRESSURE OF CO GAS IN THE GAS TO BECOME LESS AS THE TEMPERATURE IS DECREASED,
2
CAN DECREASES WHEN IT IS SOLUBLE THE GAS MOVES MORE SLOWLY, AND
OPENED
IS THUS LESS PRONE TO ESCAPING THE
P S SOLUTION. THEREFORE, ITS
SOLUBILITY IS HIGHER AT LOWER
6 TEMPERATURES