Page 13 - MSC手冊 20220831
P. 13
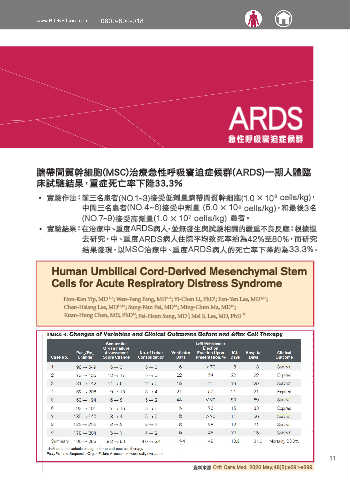
ARDS
急性呼吸窘迫症候群
臍帶間質幹細胞(MSC)治療急性呼吸窘迫症候群(ARDS)一期人體臨
床試驗結果,重症死亡率下降33.3%
˙ 實驗作法:前三名患者(NO.1~3)接受低劑量臍帶間質幹細胞(1.0 × 10 cells/kg) ,
6
中間三名患者(NO.4~6)接受中劑量 (5.0 × 10 cells/kg),和最後3名
6
(NO.7~9)接受高劑量(1.0 × 10 cells/kg) 患者。
7
˙ 實驗結果:在治療中、重度ARDS病人,並無發生與試驗相關的嚴重不良反應;根據過
去研究,中、重度 ARDS病人住院平均致死率約為42%至80%,而研究
結果發現,以MSC治療中、重度 ARDS病人的死亡率下降約為33.3%。
Human Umbilical Cord-Derived Mesenchymal Stem
Cells for Acute Respiratory Distress Syndrome
1–5
1
Hon-Kan Yip, MD ; Wen-Feng Fang, MD ; Yi-Chen Li, PhD ; Fan-Yen Lee, MD ;
6–8
9,10
Chen-Hsiang Lee, MD 11,12 ; Sung-Nan Pei, MD ; Ming-Chun Ma, MD ;
13
13
Kuan-Hung Chen, MD, PhD ; Pei-Hsun Sung, MD ; Mel S. Lee, MD, PhD 15
1
14
Downloaded from http://journals.lww.com/ccmjournal by BhDMf5ePHKbH4TTImqenVPZyxlQxTREHzMaYtY7nPvvxErytLiXzT11NQP+kn/MW on 05/02/2020
Objectives: To investigate the safety, feasibility, and possible ad- Patients: Moderate-to-severe acute respiratory distress syndrome
verse events of single-dose human umbilical cord-derived mes- with a Pao /Fio ratio less than 200.
2
2
enchymal stem cells in patients with moderate-to-severe acute Interventions: Scaling for doses was required by Taiwan Food and
respiratory distress syndrome. Drug Administration as follows: the first three patients received
Design: Prospective phase I clinical trial. low-dose human umbilical cord-derived mesenchymal stem cells
Setting: Medical center in Kaohsiung, Taiwan. (1.0 × 10 cells/kg), the next three patients with intermediate dose
6
6
(5.0 × 10 cells/kg), and the final three patients with high dose
7
1 Division of Cardiology, Department of Internal Medicine, Kaohsiung (1.0 × 10 cells/kg) between December 2017 and August 2019.
Chang Gung Memorial Hospital and Chang Gung University College of Measurements and Main Results: Nine consecutive patients
Medicine, Kaohsiung, Taiwan. were enrolled into the study. In-hospital mortality was 33.3%
2 Institute for Translational Research in Biomedicine, Kaohsiung Chang (3/9), including two with recurrent septic shock and one
Gung Memorial Hospital, Kaohsiung, Taiwan.
3 Center for Shockwave Medicine and Tissue Engineering, Kaohsiung with ventilator-induced severe pneumomediastinum and sub-
Chang Gung Memorial Hospital, Kaohsiung, Taiwan. cutaneous emphysema. No serious prespecified cell infu-
4 Department of Medical Research, China Medical University Hospital, sion-associated or treatment-related adverse events was
China Medical University, Taichung, Taiwan. identified in any patient. Serial flow-cytometric analyses of circu-
5 Department of Nursing, Asia University, Taichung, Taiwan. lating inflammatory biomarkers (CD14 CD33 /CD11b+CD16+/
+
+
dim
6 Division of Pulmonary and Critical Care Medicine, Department of Internal CD16+MPO+/CD11b+MPO+/CD14 CD33+) and mes-
Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung enchymal stem cell markers (CD26+CD45–/CD29+CD45–/
University College of Medicine, Kaohsiung, Taiwan.
7 Department of Respiratory Therapy, Kaohsiung Chang Gung Memorial CD34+CD45–/CD44+CD45–/CD73+CD45–/CD90+CD45–/
Hospital and Chang Gung University College of Medicine, Kaohsiung, CD105+CD45–/CD26+CD45–) were notably progressively
Taiwan. reduced (p for trend < 0.001), whereas the immune cell mark- 11
8 Department of Respiratory Care, Chang Gung University of Science and ers (Helper-T-cell CD3+CD4+ /Cytotoxity-T-cell CD3+CD8+ /Regulatory-T-
資料來源 Crit Care Med. 2020 May;48(5):e391-e399.
Technology, Chiayi, Taiwan. cell CD4+CD25+FOXp3+ ) were notably increased (p for trend < 0.001)
9 Division of Thoracic and Cardiovascular Surgery, Department of Sur-
Downloaded from http://journals.lww.com/ccmjournal by BhDMf5ePHKbH4TTImqenVPZyxlQxTREHzMaYtY7nPvvxErytLiXzT11NQP+kn/MW on 05/02/2020
gery, Kaohsiung Chang Gung Memorial Hospital and Chang Gung Uni- after cell infusion.
versity College of Medicine, Kaohsiung, Taiwan. Conclusions: The result of this phase I clinical trial showed that a sin-
10 Division of Cardiovascular Surgery, Department of Surgery, Tri-Service gle-dose IV infusion of human umbilical cord-derived mesenchymal
General Hospital, National Defense Medical Center, Taipei, Taiwan. stem cells was safe with favorable outcome in nine acute respiratory
11 Division of Infectious Diseases, Department of Internal Medicine, Kao- distress syndrome patients. (Crit Care Med 2020; 48:e391–e399)
hsiung Chang Gung Memorial Hospital and Chang Gung University Col-
lege of Medicine, Kaohsiung, Taiwan. Key Words: acute respiratory distress syndrome; allogenic
12 Division of Cardiology, Department of Internal Medicine, Xiamen Chang mesenchymal stem cells; inflammation; in-hospital mortality;
Gung Memorial Hospital, Fujian, China. sepsis
13 Division of Hema-Oncology, Department of Internal Medicine, Kaohsiung
Chang Gung Memorial Hospital and Chang Gung University College of
Medicine, Kaohsiung, Taiwan.
14 Department of Anesthesiology, Kaohsiung Chang Gung Memorial Hos- cute respiratory distress syndrome (ARDS), a glob-
pital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
15 Department of Orthopedics, Kaohsiung Chang Gung Memorial Hospital ally growing disease frequently complicated with mul-
and Chang Gung University College of Medicine, Kaohsiung, Taiwan. Atiple organ failure (1–6), has been reported to lead to
Copyright © 2020 by the Society of Critical Care Medicine and Wolters unacceptably high in-hospital mortality (1–7), especially in
Kluwer Health, Inc. All Rights Reserved. those of ARDS complicated by sepsis (7–11). Although the eti-
DOI: 10.1097/CCM.0000000000004285 ologies and underlying mechanisms of this disease have been
Critical Care Medicine www.ccmjournal.org e391
Copyright © 2020 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.