Page 33 - Pharmaceutics IV (02-06-01305)
P. 33
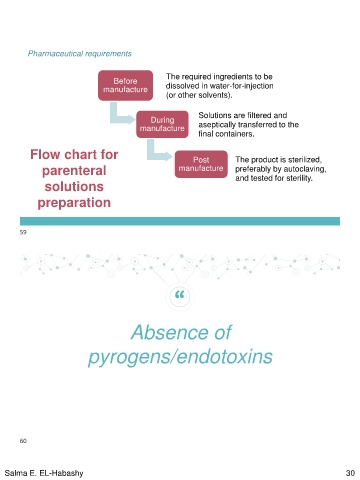
Pharmaceutical requirements
Before The required ingredients to be
manufacture dissolved in water-for-injection
(or other solvents).
During Solutions are filtered and
manufacture aseptically transferred to the
final containers.
Flow chart for Post The product is sterilized,
parenteral manufacture preferably by autoclaving,
solutions and tested for sterility.
preparation
59
“
Absence of
pyrogens/endotoxins
60 30
Salma E. EL-Habashy