Page 160 - Pharm.Org.Chem-I 02-06-05-101
P. 160
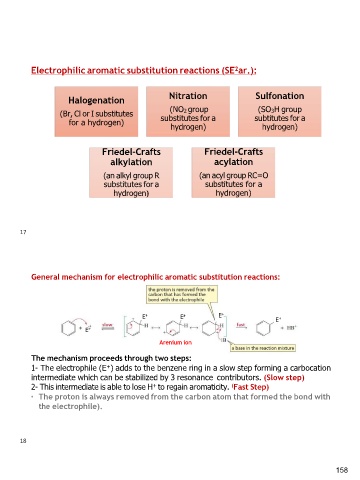
Electrophilic aromatic substitution reactions (SE2ar.):
Halogenation Nitration Sulfonation
(Br, Cl or I substitutes (NO2 group (SO3H group
for a hydrogen) substitutes for a subtitutes for a
hydrogen) hydrogen)
Friedel-Crafts Friedel-Crafts
alkylation acylation
(an alkyl group R (an acyl group RC=O
substitutes for a substitutes for a
hydrogen)
hydrogen)
17
General mechanism for electrophilic aromatic substitution reactions:
E+ E+ E+ E+
E+
Arenium ion
The mechanism proceeds through two steps:
1- The electrophile (E+) adds to the benzene ring in a slow step forming a carbocation
intermediate which can be stabilized by 3 resonance contributors. (Slow step)
2- This intermediate is able to lose H+ to regain aromaticity. (Fast Step)
• The proton is always removed from the carbon atom that formed the bond with
the electrophile).
18
158