Page 161 - Pharm.Org.Chem-I 02-06-05-101
P. 161
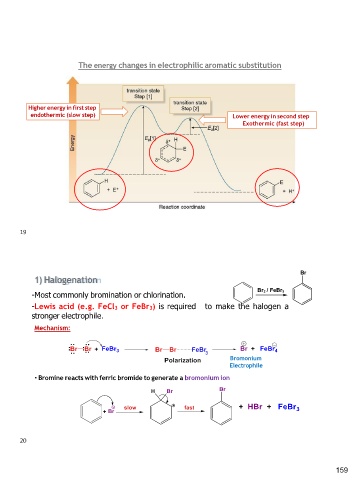
The energy changes in electrophilic aromatic substitution
Higher energy in first step Lower energy in second step
endothermic (slow step) Exothermic (fast step)
19
Br
-Most commonly bromination or chlorination. Br2 / FeBr3
-Lewis acid (e.g. FeCl3 or FeBr3) is required
stronger electrophile. to make the halogen a
Mechanism:
Bromonium
Electrophile
• Bromine reacts with ferric bromide to generate a bromonium ion
20
159