Page 58 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 58
Properties of pH meter
- This electrode is not affected by the presence of oxidizing or
reducing agents
- Operative over a wide pH range
- Responds fast and functions well in physiological systems
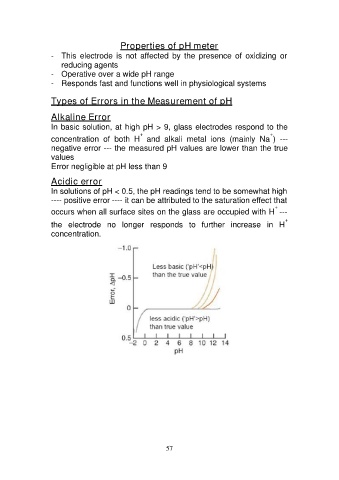
Types of Errors in the Measurement of pH
Alkaline Error
In basic solution, at high pH > 9, glass electrodes respond to the
++
concentration of both H and alkali metal ions (mainly Na ) ---
negative error --- the measured pH values are lower than the true
values
Error negligible at pH less than 9
Acidic error
In solutions of pH < 0.5, the pH readings tend to be somewhat high
---- positive error ---- it can be attributed to the saturation effect that
+
occurs when all surface sites on the glass are occupied with H ---
+
the electrode no longer responds to further increase in H
concentration.
57