Page 62 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 62
1- Monodentate ligand which has only one donor atom. The ligand is
bound to the metal ion at only one point by the donation of a lone pair of
electrons, e.g. H2O, NH3, CN -…
2- Multidentate ligand which has two or more donor atoms each of them
has a lone pair of electrons and it may be possible to form two or more
coordinate bonds with the same metal ion e.g. ethylenediamine (bidentate
ligand) and ethylenediaminetetraacetic acid, EDTA (hexadentate ligand).
Multidentate ligands are called chelating agents (from a Greek word
meaning claw), since they combine with metal ions in a manner
reminiscent of crab’s claws to form cyclic structure.
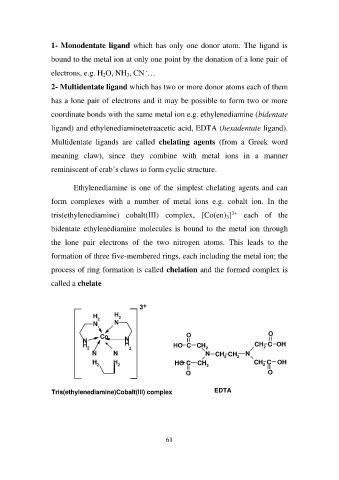
Ethylenediamine is one of the simplest chelating agents and can
form complexes with a number of metal ions e.g. cobalt ion. In the
tris(ethylenediamine) cobalt(III) complex, [Co(en)3]3+ each of the
bidentate ethylenediamine molecules is bound to the metal ion through
the lone pair electrons of the two nitrogen atoms. This leads to the
formation of three five-membered rings, each including the metal ion; the
process of ring formation is called chelation and the formed complex is
called a chelate
3+
H2 H2
N N
Co N O CH2 O
N H HO C N
H2 CH2 C OH
2 HO C CH2 N
NN O
CH2 CH2 CH2 C OH
O
H2 H2
Tris(ethylenediamine)Cobalt(III) complex EDTA
61