Page 65 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 65
The chelate effect, then, is the observation that multidentate ligands
form more stable metal complexes than do similar monodentate ligands.
It is most pronounced for ligands such as EDTA or DCTA (trans-
diaminocyclohexane-tetracetic acid), which can occupy all six
coordination sites about a metal ion.
Chemistry and properties of EDTA
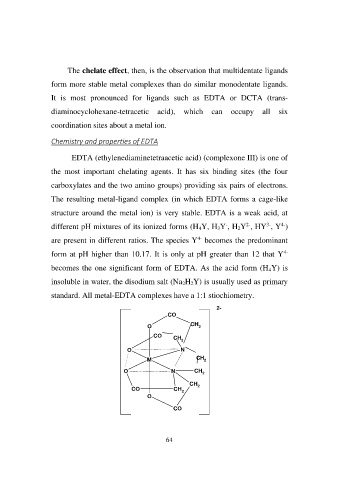
EDTA (ethylenediaminetetraacetic acid) (complexone III) is one of
the most important chelating agents. It has six binding sites (the four
carboxylates and the two amino groups) providing six pairs of electrons.
The resulting metal-ligand complex (in which EDTA forms a cage-like
structure around the metal ion) is very stable. EDTA is a weak acid, at
different pH mixtures of its ionized forms (H4Y, H3Y-, H2Y2-, HY3-, Y4-)
are present in different ratios. The species Y4- becomes the predominant
form at pH higher than 10.17. It is only at pH greater than 12 that Y4-
becomes the one significant form of EDTA. As the acid form (H4Y) is
insoluble in water, the disodium salt (Na2H2Y) is usually used as primary
standard. All metal-EDTA complexes have a 1:1 stiochiometry.
2-
CO
O CH2
CO CH2
O N
M CH2
O N CH2
CO CH2 CH2
O
CO
64