Page 68 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 68
1-3 Bi3+; Fe3+
4-6 Pb2+; Cu2+; Zn2+; Co2+; Ni2+, Mn2+; Fe2+; Al3+; Cd2+; Sn2+
8-10 Ca2+; Sr2+; Ba2+; Mg2+
(b) The effect of other complexing agents
Buffer is usually added during titration with EDTA in order to
maintain constant pH. If one of the buffer components is a complexing
agent (e.g. NH3) it will affect the stability of the EDTA complex because
the metal ion uncombined with EDTA may not be present as simple ion.
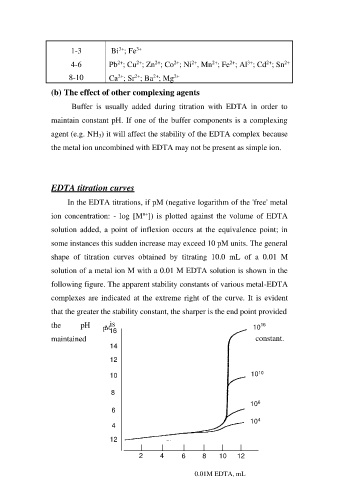
EDTA titration curves
In the EDTA titrations, if pM (negative logarithm of the 'free' metal
ion concentration: - log [Mn+]) is plotted against the volume of EDTA
solution added, a point of inflexion occurs at the equivalence point; in
some instances this sudden increase may exceed 10 pM units. The general
shape of titration curves obtained by titrating 10.0 mL of a 0.01 M
solution of a metal ion M with a 0.01 M EDTA solution is shown in the
following figure. The apparent stability constants of various metal-EDTA
complexes are indicated at the extreme right of the curve. It is evident
that the greater the stability constant, the sharper is the end point provided
the pH pM1is6 1016
maintained
14 constant.
12
10 1010
8 106
104
6
4
12 67
2 4 6 8 10 12
0.01M EDTA, mL