Page 66 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 66
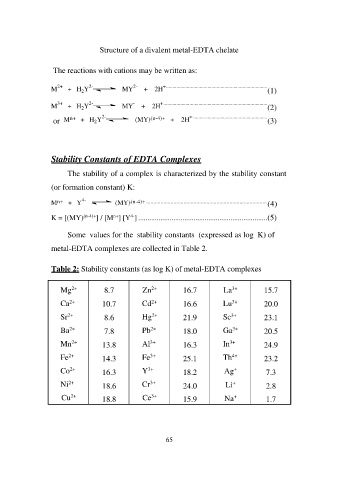
Structure of a divalent metal-EDTA chelate
The reactions with cations may be written as:
M2+ + H2Y2- (1)MY2- + 2H+..............................................................................
M3+ + H2Y2- (2)MY- + 2H+................................................................................
(MY)(n-4)+ + (3)2H+ .........................................................
or Mn+ + H2Y2-
Stability Constants of EDTA Complexes
The stability of a complex is characterized by the stability constant
(or formation constant) K:
Mn+ + Y4- (MY)(n-4)+ .................................................................................... (4)
K = [(MY)(n-4)+] / [Mn+] [Y4-] ......................................................................(5)
Some values for the stability constants (expressed as log K) of
metal-EDTA complexes are collected in Table 2.
Table 2: Stability constants (as log K) of metal-EDTA complexes
Mg2+ 8.7 Zn2+ 16.7 La3+ 15.7
Ca2+ 10.7 Cd2+ 16.6 Lu3+ 20.0
Sr2+ 8.6 Hg2+ 21.9 Sc3+ 23.1
Ba2+ 7.8 Pb2+ 18.0 Ga3+ 20.5
Mn2+ 13.8 Al3+ 16.3 In3+ 24.9
Fe2+ 14.3 Fe3+ 25.1 Th4+ 23.2
Co2+ 16.3 Y3+ 18.2 Ag+ 7.3
Ni2+ 18.6 Cr3+ 24.0 Li+ 2.8
Cu2+ 18.8 Ce3+ 15.9 Na+ 1.7
65