Page 29 - Instrumental Analysis - Pharm D Clinical- 07-PA403
P. 29
UV-visible Spectrophotometry
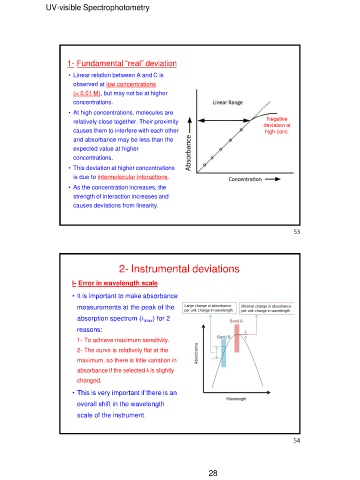
1- Fundamental “real” deviation Negative
deviation at
• Linear relation between A and C is high conc.
observed at low concentrations
(< 0.01 M), but may not be at higher 53
concentrations.
• At high concentrations, molecules are
relatively close together. Their proximity
causes them to interfere with each other
and absorbance may be less than the
expected value at higher
concentrations.
• This deviation at higher concentrations
is due to intermolecular interactions.
• As the concentration increases, the
strength of interaction increases and
causes deviations from linearity.
2- Instrumental deviations 54
I- Error in wavelength scale
• It is important to make absorbance
measurements at the peak of the
absorption spectrum (λmax) for 2
reasons:
1- To achieve maximum sensitivity.
2- The curve is relatively flat at the
maximum, so there is little variation in
absorbance if the selected λ is slightly
changed.
• This is very important if there is an
overall shift in the wavelength
scale of the instrument.
28