Page 18 - Practical-workbook-organic-3
P. 18
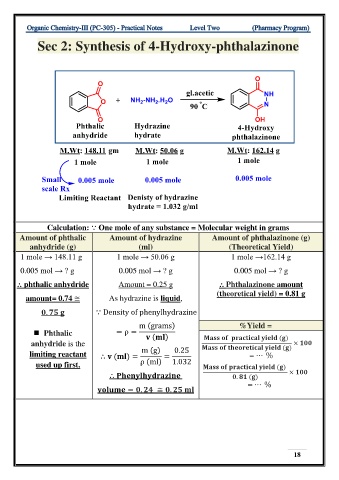
Sec 2: Synthesis of 4-Hydroxy-phthalazinone
Calculation: ⸪ One mole of any substance = Molecular weight in grams
Amount of phthalic Amount of hydrazine Amount of phthalazinone (g)
anhydride (g) (ml) (Theoretical Yield)
1 mole → 148.11 g 1 mole → 50.06 g 1 mole →162.14 g
0.005 mol → ? g 0.005 mol → ? g 0.005 mol → ? g
⸫ phthalic anhydride Amount = 0.25 g ⸫ Phthalazinone amount
(theoretical yield) = 0.81 g
amount= 0.74 ≅ As hydrazine is liquid,
. ⸪ Density of phenylhydrazine
m (grams) %Yield =
◼ Phthalic = ρ = ( ) ( )
anhydride is the ( ) ×
limiting reactant ⸫ ( ) = m (g) = 0.25 = ⋯ %
used up first. ρ (ml) 1.032 ( )
⸫ . ( ) ×
= . ≅ . = ⋯ %
18