Page 117 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 117
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
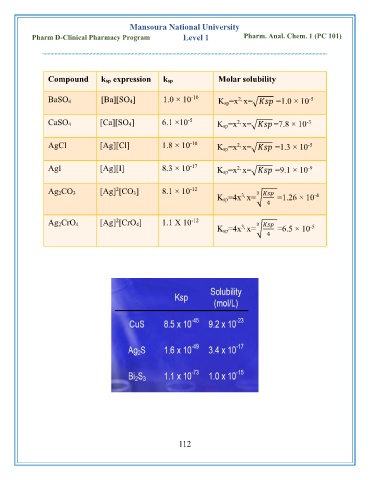
Compound ksp expression ksp Molar solubility
BaSO 4 [Ba][SO 4] 1.0 × 10 K sp=x x=√ =1.0 × 10
-10
-5
2,
-5
CaSO 4 [Ca][SO 4] 6.1 ×10 K sp=x x=√ =7.8 × 10
2,
-3
-10
AgCl [Ag][Cl] 1.8 × 10 K sp=x x=√ =1.3 × 10
-5
2,
-17
AgI [Ag][I] 8.3 × 10 K sp=x x=√ =9.1 × 10
2,
-9
2
-12
Ag 2CO 3 [Ag] [CO 3] 8.1 × 10 3
-4
3,
K sp=4x x=√ =1.26 × 10
4
2
-12
Ag 2CrO 4 [Ag] [CrO 4] 1.1 X 10 3
3,
-5
K sp=4x x=√ =6.5 × 10
4
112