Page 119 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 119
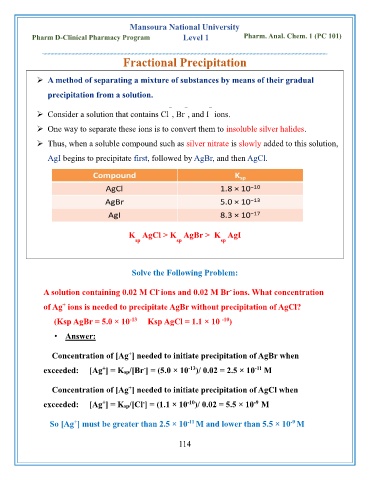
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
Fractional Precipitation
➢ A method of separating a mixture of substances by means of their gradual
precipitation from a solution.
− − −
➢ Consider a solution that contains Cl , Br , and I ions.
➢ One way to separate these ions is to convert them to insoluble silver halides.
➢ Thus, when a soluble compound such as silver nitrate is slowly added to this solution,
AgI begins to precipitate first, followed by AgBr, and then AgCl.
K AgCl > K AgBr > K AgI
sp sp sp
Solve the Following Problem:
-
-
A solution containing 0.02 M Cl ions and 0.02 M Br ions. What concentration
of Ag ions is needed to precipitate AgBr without precipitation of AgCl?
+
-10
-13
(Ksp AgBr = 5.0 × 10 Ksp AgCl = 1.1 × 10 )
• Answer:
+
Concentration of [Ag ] needed to initiate precipitation of AgBr when
-13
-
exceeded: [Ag ] = Ksp/[Br ] = (5.0 × 10 )/ 0.02 = 2.5 × 10 M
-11
+
+
Concentration of [Ag ] needed to initiate precipitation of AgCl when
-10
-9
-
+
exceeded: [Ag ] = Ksp/[Cl ] = (1.1 × 10 )/ 0.02 = 5.5 × 10 M
-9
+
-11
So [Ag ] must be greater than 2.5 × 10 M and lower than 5.5 × 10 M
114