Page 124 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 124
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
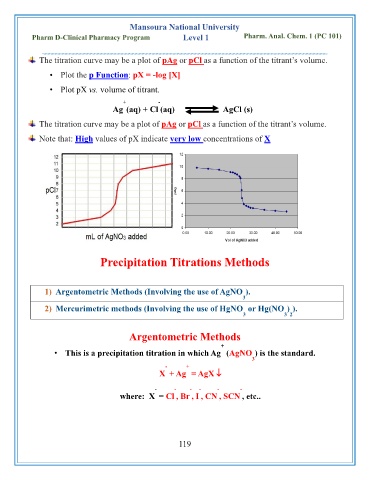
The titration curve may be a plot of pAg or pCl as a function of the titrant’s volume.
• Plot the p Function: pX = -log [X]
• Plot pX vs. volume of titrant.
+ -
Ag (aq) + Cl (aq) AgCl (s)
The titration curve may be a plot of pAg or pCl as a function of the titrant’s volume.
Note that: High values of pX indicate very low concentrations of X
Precipitation Titrations Methods
1) Argentometric Methods (Involving the use of AgNO ).
3
2) Mercurimetric methods (Involving the use of HgNO or Hg(NO ) ).
3 3 2
Argentometric Methods
+
• This is a precipitation titration in which Ag (AgNO ) is the standard.
3
- +
X + Ag = AgX
- - - - - -
where: X = Cl , Br , I , CN , SCN , etc..
119