Page 128 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 128
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
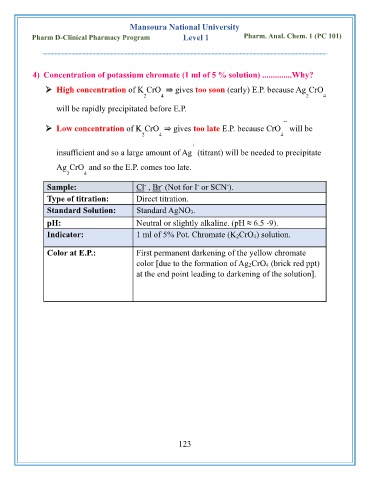
4) Concentration of potassium chromate (1 ml of 5 % solution) ..............Why?
➢ High concentration of K CrO ⇒ gives too soon (early) E.P. because Ag CrO
2 4 2 4
will be rapidly precipitated before E.P.
--
➢ Low concentration of K CrO ⇒ gives too late E.P. because CrO will be
2 4 4
+
insufficient and so a large amount of Ag (titrant) will be needed to precipitate
Ag CrO and so the E.P. comes too late.
2 4
-
-
-
-
Sample: Cl , Br (Not for I or SCN ).
Type of titration: Direct titration.
Standard Solution: Standard AgNO 3.
pH: Neutral or slightly alkaline. (pH ≈ 6.5 -9).
Indicator: 1 ml of 5% Pot. Chromate (K 2CrO 4) solution.
Color at E.P.: First permanent darkening of the yellow chromate
color [due to the formation of Ag 2CrO 4 (brick red ppt)
at the end point leading to darkening of the solution].
123