Page 10 - Professorial Lecture - Prof Oyedele
P. 10
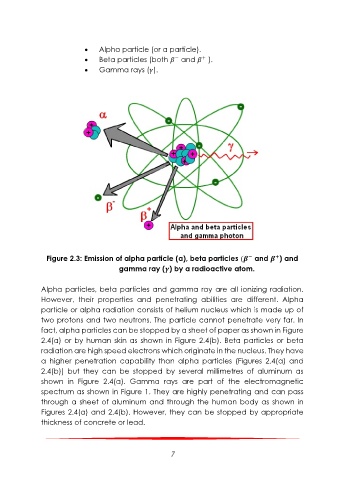
Alpha particle (or α particle).
−
+
Beta particles (both and ).
Gamma rays ( ).
Figure 2.3: Emission of alpha particle (α), beta particles ( and ) and
+
−
gamma ray ( ) by a radioactive atom.
Alpha particles, beta particles and gamma ray are all ionizing radiation.
However, their properties and penetrating abilities are different. Alpha
particle or alpha radiation consists of helium nucleus which is made up of
two protons and two neutrons. The particle cannot penetrate very far. In
fact, alpha particles can be stopped by a sheet of paper as shown in Figure
2.4(a) or by human skin as shown in Figure 2.4(b). Beta particles or beta
radiation are high speed electrons which originate in the nucleus. They have
a higher penetration capability than alpha particles (Figures 2.4(a) and
2.4(b)) but they can be stopped by several millimetres of aluminum as
shown in Figure 2.4(a). Gamma rays are part of the electromagnetic
spectrum as shown in Figure 1. They are highly penetrating and can pass
through a sheet of aluminum and through the human body as shown in
Figures 2.4(a) and 2.4(b). However, they can be stopped by appropriate
thickness of concrete or lead.