Page 11 - TNT_placeholder
P. 11
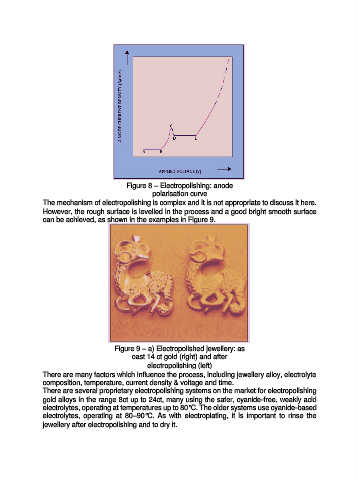
Figure 8 – Electropolishing: anode
polarisation curve
The mechanism of electropolishing is complex and it is not appropriate to discuss it here.
However, the rough surface is levelled in the process and a good bright smooth surface
can be achieved, as shown in the examples in Figure 9.
Figure 9 – a) Electropolished jewellery: as
cast 14 ct gold (right) and after
electropolishing (left)
There are many factors which influence the process, including jewellery alloy, electrolyte
composition, temperature, current density & voltage and time.
There are several proprietary electropolishing systems on the market for electropolishing
gold alloys in the range 8ct up to 24ct, many using the safer, cyanide-free, weakly acid
electrolytes, operating at temperatures up to 80°C. The older systems use cyanide-based
electrolytes, operating at 80–90°C. As with electroplating, it is important to rinse the
jewellery after electropolishing and to dry it.