Page 2 - Illustrated Pathology of the Bone Marrow
P. 2
Disease-specific changes after therapy 125
Table 14.1. Bone marrow changes in the three to four weeks
following myeloablative therapy.
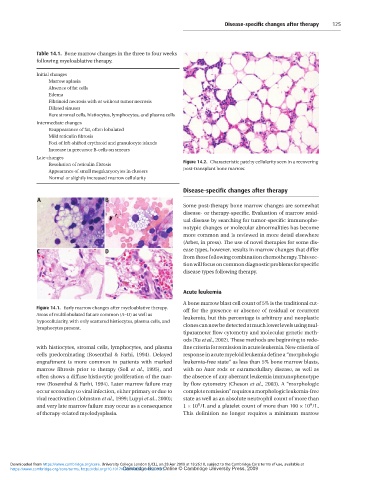
Initial changes Figure 14.2. Characteristic patchy cellularity seen in a recovering
Marrow aplasia post-transplant bone marrow.
Absence of fat cells
Edema
Fibrinoid necrosis with or without tumor necrosis
Dilated sinuses
Rare stromal cells, histiocytes, lymphocytes, and plasma cells
Intermediate changes
Reappearance of fat, often lobulated
Mild reticulin fibrosis
Foci of left-shifted erythroid and granulocyte islands
Increase in precursor B-cells on smears
Late changes
Resolution of reticulin fibrosis
Appearance of small megakaryocytes in clusters
Normal or slightly increased marrow cellularity
AB Disease-specific changes after therapy
CD
Some post-therapy bone marrow changes are somewhat
disease- or therapy-specific. Evaluation of marrow resid-
ual disease by searching for tumor-specific immunophe-
notypic changes or molecular abnormalities has become
more common and is reviewed in more detail elsewhere
(Arber, in press). The use of novel therapies for some dis-
ease types, however, results in marrow changes that differ
from those following combination chemotherapy. This sec-
tion will focus on common diagnostic problems for specific
disease types following therapy.
Figure 14.1. Early marrow changes after myeloablative therapy. Acute leukemia
Areas of multilobulated fat are common (A–D) as well as
hypocellularity, with only scattered histiocytes, plasma cells, and A bone marrow blast cell count of 5% is the traditional cut-
lymphocytes present. off for the presence or absence of residual or recurrent
leukemia, but this percentage is arbitrary and neoplastic
with histiocytes, stromal cells, lymphocytes, and plasma clones can now be detected at much lower levels using mul-
cells predominating (Rosenthal & Farhi, 1994). Delayed tiparameter flow cytometry and molecular genetic meth-
engraftment is more common in patients with marked ods (Xu et al., 2002). These methods are beginning to rede-
marrow fibrosis prior to therapy (Soll et al., 1995), and fine criteria for remission in acute leukemia. New criteria of
often shows a diffuse histiocytic proliferation of the mar- response in acute myeloid leukemia define a “morphologic
row (Rosenthal & Farhi, 1994). Later marrow failure may leukemia-free state” as less than 5% bone marrow blasts,
occur secondary to viral infection, either primary or due to with no Auer rods or extramedullary disease, as well as
viral reactivation (Johnston et al., 1999; Luppi et al., 2000); the absence of any aberrant leukemia immunophenotype
and very late marrow failure may occur as a consequence by flow cytometry (Cheson et al., 2003). A “morphologic
of therapy-related myelodysplasia. complete remission” requires a morphologic leukemia-free
state as well as an absolute neutrophil count of more than
1 × 109/L and a platelet count of more than 100 × 109/L.
This definition no longer requires a minimum marrow
Downloaded from https://www.cambridge.org/core. University College London (UCL), on 28 Apr 2018 at 18:26:18, subject to the Cambridge Core terms of use, available at
https://www.cambridge.org/core/terms. https://doi.org/10.1017/CCBaOm97b8r0i5d1g1e54B35o3o1k.0s15Online © Cambridge University Press, 2009