Page 6 - Illustrated Pathology of the Bone Marrow
P. 6
Fibrosis 129
usually do not show perinuclear cytoplasmic clearing, and AB
demonstrate Auer rods that are not present in reactive
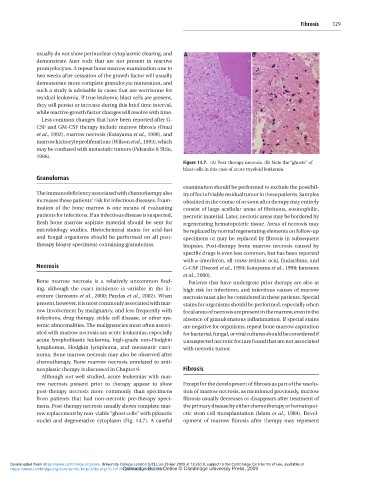
promyelocytes. A repeat bone marrow examination one to Figure 14.7. (A) Post-therapy necrosis. (B) Note the “ghosts” of
two weeks after cessation of the growth factor will usually blast cells in this case of acute myeloid leukemia.
demonstrate more complete granulocyte maturation, and
such a study is advisable in cases that are worrisome for examination should be performed to exclude the possibil-
residual leukemia. If true leukemic blast cells are present, ity of foci of viable residual tumor in these patients. Samples
they will persist or increase during this brief time interval, obtained in the course of or soon after therapy may entirely
while reactive growth factor changes will resolve with time. consist of large acellular areas of fibrinous, eosinophilic,
necrotic material. Later, necrotic areas may be bordered by
Less common changes that have been reported after G- regenerating hematopoietic tissue. Areas of necrosis may
CSF and GM-CSF therapy include marrow fibrosis (Orazi be replaced by normal regenerating elements on follow-up
et al., 1992), marrow necrosis (Katayama et al., 1998), and specimens or may be replaced by fibrosis in subsequent
marrow histiocyte proliferations (Wilson et al., 1993), which biopsies. Post-therapy bone marrow necrosis caused by
may be confused with metastatic tumors (Pekarske & Shin, specific drugs is even less common, but has been reported
1996). with α-interferon, all-trans-retinoic acid, fludaribine, and
G-CSF (Dreosti et al., 1994; Katayama et al., 1998; Janssens
Granulomas et al., 2000).
The immunodeficiency associated with chemotherapy also Patients that have undergone prior therapy are also at
increases these patients’ risk for infectious diseases. Exam- high risk for infections, and infectious causes of marrow
ination of the bone marrow is one means of evaluating necrosis must also be considered in these patients. Special
patients for infections. If an infectious disease is suspected, stains for organisms should be performed, especially when
fresh bone marrow aspirate material should be sent for focal areas of necrosis are present in the marrow, even in the
microbiology studies. Histochemical stains for acid-fast absence of granulomatous inflammation. If special stains
and fungal organisms should be performed on all post- are negative for organisms, repeat bone marrow aspiration
therapy biopsy specimens containing granulomas. for bacterial, fungal, or viral cultures should be considered if
unsuspected necrotic foci are found that are not associated
Necrosis with necrotic tumor.
Bone marrow necrosis is a relatively uncommon find- Fibrosis
ing, although the exact incidence is variable in the lit-
erature (Janssens et al., 2000; Paydas et al., 2002). When Except for the development of fibrosis as part of the resolu-
present, however, it is most commonly associated with mar- tion of marrow necrosis, as mentioned previously, marrow
row involvement by malignancy, and less frequently with fibrosis usually decreases or disappears after treatment of
infections, drug therapy, sickle cell disease, or other sys- the primary disease by either chemotherapy or hematopoi-
temic abnormalities. The malignancies most often associ- etic stem cell transplantation (Islam et al., 1984). Devel-
ated with marrow necrosis are acute leukemias, especially opment of marrow fibrosis after therapy may represent
acute lymphoblastic leukemia, high-grade non-Hodgkin
lymphomas, Hodgkin lymphoma, and metastatic carci-
noma. Bone marrow necrosis may also be observed after
chemotherapy. Bone marrow necrosis unrelated to anti-
neoplastic therapy is discussed in Chapter 6.
Although not well studied, acute leukemias with mar-
row necrosis present prior to therapy appear to show
post-therapy necrosis more commonly than specimens
from patients that had non-necrotic pre-therapy speci-
mens. Post-therapy necrosis usually shows complete mar-
row replacement by non-viable “ghost cells” with pyknotic
nuclei and degenerative cytoplasm (Fig. 14.7). A careful
Downloaded from https://www.cambridge.org/core. University College London (UCL), on 28 Apr 2018 at 18:26:18, subject to the Cambridge Core terms of use, available at
https://www.cambridge.org/core/terms. https://doi.org/10.1017/CCBaOm97b8r0i5d1g1e54B35o3o1k.0s15Online © Cambridge University Press, 2009