Page 4 - Illustrated Pathology of the Bone Marrow
P. 4
Disease-specific changes after therapy 127
that cell maturation is occurring and to confirm loss of the
characteristic t(15;17) of this disease.
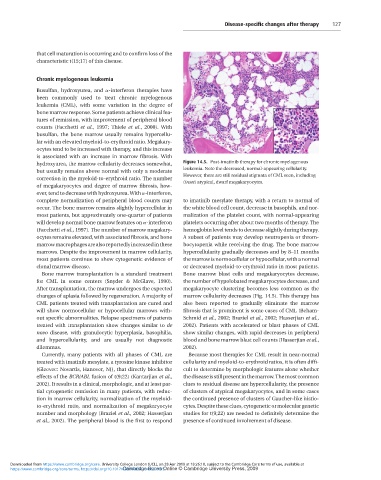
Chronic myelogenous leukemia Figure 14.5. Post-imatinib therapy for chronic myelogenous
leukemia. Note the decreased, normal-appearing cellularity.
Busulfan, hydroxyurea, and α-interferon therapies have However, there are still residual stigmata of CML seen, including
been commonly used to treat chronic myelogenous (inset) atypical, dwarf megakaryocytes.
leukemia (CML), with some variation in the degree of
bone marrow response. Some patients achieve clinical fea- to imatinib mesylate therapy, with a return to normal of
tures of remission, with improvement of peripheral blood the white blood cell count, decrease in basophils, and nor-
counts (Facchetti et al., 1997; Thiele et al., 2000). With malization of the platelet count, with normal-appearing
busulfan, the bone marrow usually remains hypercellu- platelets occurring after about two months of therapy. The
lar with an elevated myeloid-to-erythroid ratio. Megakary- hemoglobin level tends to decrease slightly during therapy.
ocytes tend to be increased with therapy, and this increase A subset of patients may develop neutropenia or throm-
is associated with an increase in marrow fibrosis. With bocytopenia while receiving the drug. The bone marrow
hydroxyurea, the marrow cellularity decreases somewhat, hypercellularity gradually decreases and by 8–11 months
but usually remains above normal with only a moderate the marrow is normocellular or hypocellular, with a normal
correction in the myeloid-to-erythroid ratio. The number or decreased myeloid-to-erythroid ratio in most patients.
of megakaryocytes and degree of marrow fibrosis, how- Bone marrow blast cells and megakaryocytes decrease,
ever, tend to decrease with hydroxyurea. With α-interferon, the number of hypolobated megakaryocytes decrease, and
complete normalization of peripheral blood counts may megakaryocyte clustering becomes less common as the
occur. The bone marrow remains slightly hypercellular in marrow cellularity decreases (Fig. 14.5). This therapy has
most patients, but approximately one-quarter of patients also been reported to gradually eliminate the marrow
will develop normal bone marrow features on α-interferon fibrosis that is prominent is some cases of CML (Beham-
(Facchetti et al., 1997). The number of marrow megakary- Schmid et al., 2002; Braziel et al., 2002; Hasserjian et al.,
ocytes remains elevated, with associated fibrosis, and bone 2002). Patients with accelerated or blast phases of CML
marrow macrophages are also reportedly increased in these show similar changes, with rapid decreases in peripheral
marrows. Despite the improvement in marrow cellularity, blood and bone marrow blast cell counts (Hasserjian et al.,
most patients continue to show cytogenetic evidence of 2002).
clonal marrow disease.
Because most therapies for CML result in near-normal
Bone marrow transplantation is a standard treatment cellularity and myeloid-to-erythroid ratios, it is often diffi-
for CML in some centers (Snyder & McGlave, 1990). cult to determine by morphologic features alone whether
After transplantation, the marrow undergoes the expected the disease is still present in the marrow. The most common
changes of aplasia followed by regeneration. A majority of clues to residual disease are hypercellularity, the presence
CML patients treated with transplantation are cured and of clusters of atypical megakaryocytes, and in some cases
will show normocellular or hypocellular marrows with- the continued presence of clusters of Gaucher-like histio-
out specific abnormalities. Relapse specimens of patients cytes. Despite these clues, cytogenetic or molecular genetic
treated with transplantation show changes similar to de studies for t(9;22) are needed to definitely determine the
novo disease, with granulocytic hyperplasia, basophilia, presence of continued involvement of disease.
and hypercellularity, and are usually not diagnostic
dilemmas.
Currently, many patients with all phases of CML are
treated with imatinib mesylate, a tyrosine kinase inhibitor
(Gleevec: Novartis, Hanover, NJ), that directly blocks the
effects of the BCR/ABL fusion of t(9;22) (Kantarjian et al.,
2002). It results in a clinical, morphologic, and at least par-
tial cytogenetic remission in many patients, with reduc-
tion in marrow cellularity, normalization of the myeloid-
to-erythroid ratio, and normalization of megakaryocyte
number and morphology (Braziel et al., 2002; Hasserjian
et al., 2002). The peripheral blood is the first to respond
Downloaded from https://www.cambridge.org/core. University College London (UCL), on 28 Apr 2018 at 18:26:18, subject to the Cambridge Core terms of use, available at
https://www.cambridge.org/core/terms. https://doi.org/10.1017/CCBaOm97b8r0i5d1g1e54B35o3o1k.0s15Online © Cambridge University Press, 2009