Page 5 - Illustrated Pathology of the Bone Marrow
P. 5
128 Post-therapy bone marrow changes
Table 14.3. Changes associated with recombinant AB
granulocyte colony-stimulating growth factor (G-CSF)
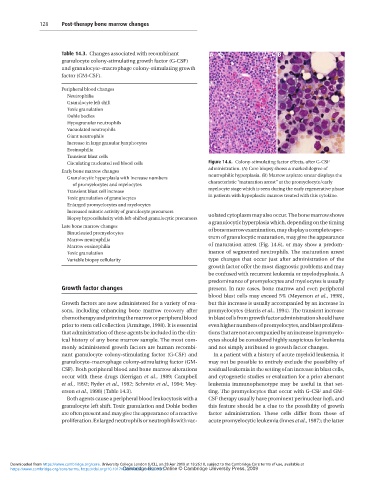
and granulocyte–macrophage colony-stimulating growth Figure 14.6. Colony-stimulating factor effects, after G-CSF
factor (GM-CSF). administration. (A) Core biopsy shows a marked degree of
neutrophilic hyperplasia. (B) Marrow aspirate smear displays the
Peripheral blood changes characteristic “maturation arrest” at the promyelocyte/early
Neutrophilia myelocyte stage which is seen during the early regenerative phase
Granulocyte left shift in patients with hypoplastic marrow treated with this cytokine.
Toxic granulation
Dohle bodies uolated cytoplasm may also occur. The bone marrow shows
Hypogranular neutrophils a granulocytic hyperplasia which, depending on the timing
Vacuolated neutrophils of bone marrow examination, may display a complete spec-
Giant neutrophils trum of granulocytic maturation, may give the appearance
Increase in large granular lymphocytes of maturation arrest (Fig. 14.6), or may show a predom-
Eosinophilia inance of segmented neutrophils. The maturation arrest
Transient blast cells type changes that occur just after administration of the
Circulating nucleated red blood cells growth factor offer the most diagnostic problems and may
be confused with recurrent leukemia or myelodysplasia. A
Early bone marrow changes predominance of promyelocytes and myelocytes is usually
Granulocytic hyperplasia with increase numbers present. In rare cases, bone marrow and even peripheral
of promyelocytes and myelocytes blood blast cells may exceed 5% (Meyerson et al., 1998),
Transient blast cell increase but this increase is usually accompanied by an increase in
Toxic granulation of granulocytes promyelocytes (Harris et al., 1994). The transient increase
Enlarged promyelocytes and myelocytes in blast cells from growth factor administration should have
Increased mitotic activity of granulocyte precursors even higher numbers of promyelocytes, and blast prolifera-
Biopsy hypocellularity with left-shifted granulocytic precursors tions that are not accompanied by an increase in promyelo-
cytes should be considered highly suspicious for leukemia
Late bone marrow changes and not simply attributed to growth factor changes.
Binucleated promyelocytes
Marrow neutrophilia In a patient with a history of acute myeloid leukemia, it
Marrow eosinophilia may not be possible to entirely exclude the possibility of
Toxic granulation residual leukemia in the setting of an increase in blast cells,
Variable biopsy cellularity and cytogenetic studies or evaluation for a prior aberrant
leukemia immunophenotype may be useful in that set-
Growth factor changes ting. The promyelocytes that occur with G-CSF and GM-
CSF therapy usually have prominent perinuclear hofs, and
Growth factors are now administered for a variety of rea- this feature should be a clue to the possibility of growth
sons, including enhancing bone marrow recovery after factor administration. These cells differ from those of
chemotherapy and priming the marrow or peripheral blood acute promyelocytic leukemia (Innes et al., 1987); the latter
prior to stem cell collection (Armitage, 1998). It is essential
that administration of these agents be included in the clin-
ical history of any bone marrow sample. The most com-
monly administered growth factors are human recombi-
nant granulocyte colony-stimulating factor (G-CSF) and
granulocyte–macrophage colony-stimulating factor (GM-
CSF). Both peripheral blood and bone marrow alterations
occur with these drugs (Kerrigan et al., 1989; Campbell
et al., 1992; Ryder et al., 1992; Schmitz et al., 1994; Mey-
erson et al., 1998) (Table 14.3).
Both agents cause a peripheral blood leukocytosis with a
granulocyte left shift. Toxic granulation and Dohle bodies
are often present and may give the appearance of a reactive
proliferation. Enlarged neutrophils or neutrophils with vac-
Downloaded from https://www.cambridge.org/core. University College London (UCL), on 28 Apr 2018 at 18:26:18, subject to the Cambridge Core terms of use, available at
https://www.cambridge.org/core/terms. https://doi.org/10.1017/CCBaOm97b8r0i5d1g1e54B35o3o1k.0s15Online © Cambridge University Press, 2009