Page 51 - PG 504-theoretical notes-phyto-1-2024-2025..
P. 51
Clinical pharmacy PharmD program Third level Phytochemistry-1 (PG-504)
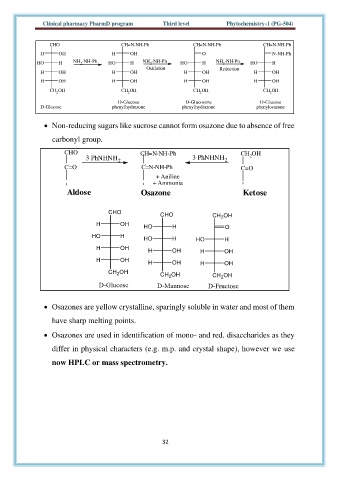
CHO CH=N-NH-Ph CH=N-NH-Ph CH=N-NH-Ph
H OH H OH O N-NH-Ph
HO H NH -NH-Ph HO H NH -NH-Ph HO H NH -NH-Ph HO H
2
2
2
Oxidation Reduction
H OH H OH H OH H OH
H OH H OH H OH H OH
CH OH CH OH CH OH CH OH
2
2
2
2
D-Glucose D-Glucosone D-Glucose
D-Glucose phenylhydrazone phenylhydrazone phenylosazone
• Non-reducing sugars like sucrose cannot form osazone due to absence of free
carbonyl group.
CHO CH=N-NH-Ph CH OH
3 PhNHNH 2 3 PhNHNH 2 2
C=O C=N-NH-Ph C=O
+ Aniline
+ Ammonia
Aldose Osazone Ketose
• Osazones are yellow crystalline, sparingly soluble in water and most of them
have sharp melting points.
• Osazones are used in identification of mono- and red. disaccharides as they
differ in physical characters (e.g. m.p. and crystal shape), however we use
now HPLC or mass spectrometry.
32