Page 31 - MEMENTO THERAPEUTIQUE RCP 2024
P. 31
DE/H/3682/001/IA/020_approved_common_SPC
In a clinical study for combined dorzolamide/timolol preservative free formulation the
observed adverse reactions have been consistent with those that were reported previously with
combined dorzolamide/timolol preserved formulation, dorzolamide hydrochloride and/or
timolol maleate.
During clinical studies, 1035 patients were treated with combined dorzolamide/timolol
preserved formulation. Approximately 2.4% of all patients discontinued therapy with
combined dorzolamide/timolol preserved formulation because of local ocular adverse
reactions; approximately 1.2% of all patients discontinued because of local adverse reactions
suggestive of allergy or hypersensitivity (such as lid inflammation and conjunctivitis).
Combined dorzolamide/timolol preservative free formulation has been shown to have a
similar safety profile to combined dorzolamide/timolol preserved formulation in a repeat
dose, double-masked, comparative study.
Timolol is absorbed into the systemic circulation. This may cause similar undesirable effects
as seen with systemic beta-blocking agents. Incidence of systemic ADRs after topical
ophthalmic administration is lower than for systemic administration.
The following adverse reactions have been reported with combined dorzolamide/timolol
preservative free formulation or one of its components either during clinical trials or during
post-marketing experience:
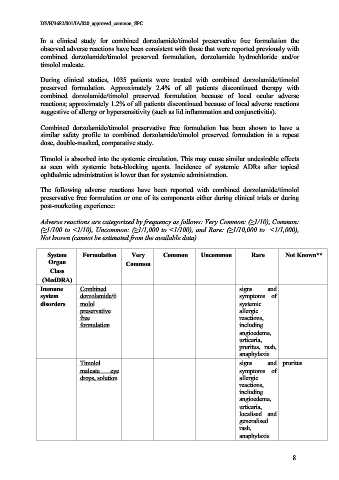
Adverse reactions are categorized by frequency as follows: Very Common: (≥1/10), Common:
(≥1/100 to <1/10), Uncommon: (≥1/1,000 to <1/100), and Rare: (≥1/10,000 to <1/1,000),
Not known (cannot be estimated from the available data).
System Formulation Very Common Uncommon Rare Not Known**
Organ Common
Class
(MedDRA)
Immune Combined signs and
system dorzolamide/ti symptoms of
disorders molol systemic
preservative allergic
free reactions,
formulation including
angioedema,
urticaria,
pruritus, rash,
anaphylaxis
Timolol signs and pruritus
maleate eye symptoms of
drops, solution allergic
reactions,
including
angioedema,
urticaria,
localised and
generalised
rash,
anaphylaxis
8