Page 34 - MEMENTO THERAPEUTIQUE RCP 2024
P. 34
DE/H/3682/001/IA/020_approved_common_SPC
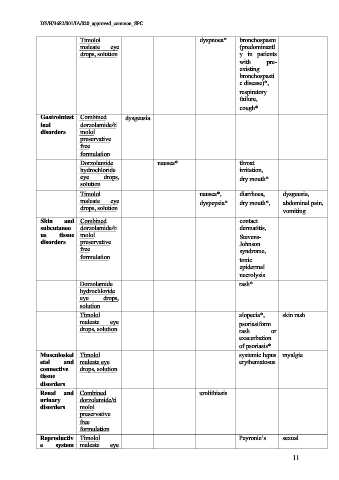
Timolol dyspnoea* bronchospasm
maleate eye (predominantl
drops, solution y in patients
with pre-
existing
bronchospasti
c disease)*,
respiratory
failure,
cough*
Gastrointest Combined dysgeusia
inal dorzolamide/ti
disorders molol
preservative
free
formulation
Dorzolamide nausea* throat
hydrochloride irritation,
eye drops, dry mouth*
solution
Timolol nausea*, diarrhoea, dysgeusia,
maleate eye dyspepsia* dry mouth*, abdominal pain,
drops, solution
vomiting
Skin and Combined contact
subcutaneo dorzolamide/ti dermatitis,
us tissue molol Stevens-
disorders preservative Johnson
free syndrome,
formulation toxic
epidermal
necrolysis
Dorzolamide rash*
hydrochloride
eye drops,
solution
Timolol alopecia*, skin rash
maleate eye psoriasiform
drops, solution rash or
exacerbation
of psoriasis*
Musculoskel Timolol systemic lupus myalgia
etal and maleate eye erythematosus
connective drops, solution
tissue
disorders
Renal and Combined urolithiasis
urinary dorzolamide/ti
disorders molol
preservative
free
formulation
Reproductiv Timolol Peyronie’s sexual
e system maleate eye
11