Page 35 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 35
Al-Khawaga and Abdelalim Stem Cell Research & Therapy (2020) 11:437 Page 16 of 33
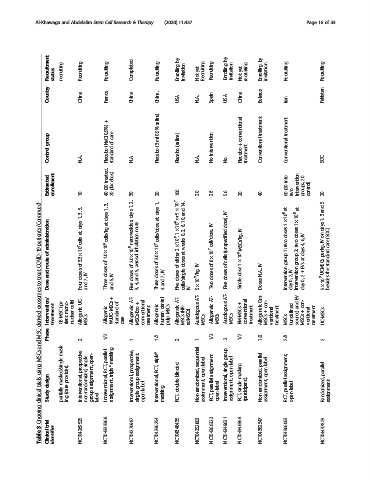
Recruitment status recruiting Recruiting Recruiting Completed Recruiting by Enrolling invitation yet Not Recruiting Recruiting by Enrolling invitation yet Not recruiting by Enrolling invitation Recruiting Recruiting
Country China France China China. USA N.A. Spain USA China Belarus Iran Pakistan
saline)
+
0.9%) 0.9% conventional treatment treatment
group (NaCl care of (3 ml (saline) intervention +
Control N.A. Placebo standard N.A. Placebo Placebo N.A. No No Placebo treatment Conventional Conventional SOC
Estimated enrollment 10 treated, (20 40 placebos) 20 30 20 100 20 26 56 20 40 into (20 60 two intervention 20 groups, control) 20
2, 1, 1, 5 × 10 7 14, 5
(Continued) 5, 3, 1, days 1, days at days days at, or and 10, 6, IV dose), at 1 × 10 8 at 1 × 10 8 and 3 1,
3,
patients administration at, cells/kg nanovesicles, route inhalation cells/dose 2 × 10 8 ,1 × 10 8 2, 0, weeks IV cell/dose, (unspecified IV MSCs/kg, doses two doses two IV 6, 4, days on IV kg, (SOC) care
COVID-19 of route 3.3 × 10 7 cells of 1.0 × 10 6 of 2.0 × 10 8 aerosol 3.0 × 10 7 of either at doses IV 8 × 10 7 of cells 1 × 10 6 IV 1: group 2: group days at EVs per standard
treat and doses IV doses IV of doses 5, and doses IV 7, of doses doses of doses dose N.A., Intervention IV 2, Intervention + 2 UCMSCs the
to Dose Four 7, and Three 5, and Five 4, 3, Three and 4 Five cells/single IV 5×10 5 /kg, Two Five Single Doses 0, day 0, day 5 × 10 5 besides
exosomes Intervention/ (pa- mono- cells) UC- + of AT- + dental MSCs AT- (HB- AT- AT- AT- + Om- con- + EV- and con- +
MSC-derived Phase treatment CB-MSC tient nuclear Allogenic MSCs Allogenic WJUC-MSCs standard care Allogeneic MSCs-Exo conventional treatment Allogeneic human pulp Allogeneic MSCs adMSCs) Autologous MSCs Allogeneic MSCs Autologous MSCs BM-MSCs conventional treatment Allogeneic MSCs ventional treatment MSCs (undefined source) MSCs ventional treatment UC-MSCs
and 2 1/2 1 1/2 2 1 1/2 2 1/2 1/2 2/3 2
MSCs mask- prospective single open- parallel masking prospective, triple* sequential group parallel label
using masked/single provider). RCT, triple* assignment, RCT, open-label assignment single open-label masking open assignment, parallel
trials design Interventional, non-randomized, assignment, Interventional, Interventional, group Interventional, double-blinded Non-randomized, parallel Interventional, single Non-randomized, parallel
clinical Study partially (care ing group label. assignment, single open-label masking RCT, assignment, RCT, open-label assignment, RCT, (participant). assignment, RCT, open-label Randomized, assignment
Ongoing
3 trial NCT04269525 NCT04333368 NCT04276987 NCT04336254 NCT04348435 NCT04352803 NCT04366323 NCT04349631 NCT04346368 NCT04382547 NCT04366063 NCT04437823
Table Clinical identifier