Page 33 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 33
Al-Khawaga and Abdelalim Stem Cell Research & Therapy (2020) 11:437 Page 14 of 33
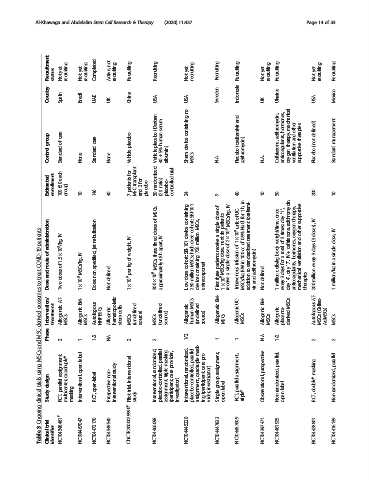
Recruitment status yet Not recruiting yet Not recruiting Completed not Active, recruiting Recruiting Recruiting yet Not recruiting Recruiting Recruiting yet Not recruiting Recruiting yet Not recruiting Recruiting
Country Spain Brazil UAE UK China USA USA Sweden Indonesia UK Ukraine USA Mexico
no
(Dextran serum containing and azithromycin, hormones, mechanical other defined)
group care of care placebo placebo human device (oseltamivir therapy, and therapies (not management
Control Standard None Standard None Vehicle Vehicle 40 + 5% albumin) trial. Sham MSCs N.A. Placebo azithromycin) N.A. Ceftriaxone, anticoagulants, oxygen ventilation supportive Placebo Standard
Estimated enrollment each (50 100 group) 10 146 40 for patients transplant MSC for 3 and placebo randomized 30 ratio) (2:1 placebo- controlled 24 40 10 30 200 10
9
7
in
IV
MSCs, of containing SBI-101 of dose patients MSCs/kg, UC- of 1 h, for (oseltami- once “1”, day azithromycin, therapy, supportive IV
patients administration IV nebulization jet IV doses fixed IV apart, device cohort: dose MSCs, million single a receive six next 2 × 10 6 of unit 1 × 10 6 of NaCl 0.9% of treatment weight/time, times: 3 of ceftriaxone, oxygen other and doses), (3 IV dose,
COVID-19 of route 1.5 × 10 6 /kg, of IV MSCs/kg, specified, weight, of kg three MSCs, 48 h SBI-101 cohort: high MSCs; 750 containing patients dose, dose single infusion 100 cc in standardized azithromycin) body cells/kg total a for + IV “7”, hormones, ventilation 3 days every single a in
treat and doses non defined per approximately dose million extracorporeal three MSCs/kg a Intravenous BW to defined 3 days day “4”, anticoagulants, mechanical million million/kg
to Dose Two 1×10 6 Dose: Not 1×10 6 300 × 10 6 Low 250 device First 1×10 6 receive MSCs/kg addition and vir Not million 1 every day therapies 200 1
exosomes Intervention/ AT- BM- hematopoietic cells MSCs BM- UC- BM- MSCs AT- (Celltex-
MSC-derived Phase treatment Allogeneic MSCs Allogenic MSCs Autologous NHPBSCs Allogenic stem MSCs (undefined source) MSCs (undefined source) Allogeneic human (undefined source) Allogeneic MSCs Allogenic MSCs Allogenic MSCs Allogenic placenta- derived Autologous MSCs AdMSCs) MSCs
and 2 1 1/2 NA 2 2 1/2 1 1 N.A 1/2 2 2
MSCs label randomized, parallel masking provider, randomized, parallel mask- pro- prospective parallel, parallel
using assignment, quadruple* open non- study interventional triple care quadruple care assignment, assignment, masking
trials design parallel Interventional, open-label interventional trial, Interventional, placebo-controlled, Interventional, placebo-controlled, (participant, investigator) group label parallel Observational, Non-randomized, label double* Non-randomized,
clinical Study RCT, multicenter, masking RCT, Prospective Pilot study assignment, (participant, investigator) assignment, ing vider, Single open RCT, triple* open RCT,
Ongoing
3 trial NCT04348461 # NCT04467047 NCT04473170 NCT04349540 ChiCTR2000029990 # NCT04466098 NCT04445220 NCT04447833 NCT04457609 NCT04397471 NCT04461925 NCT04428801 NCT04416139
Table Clinical identifier