Page 29 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 29
Al-Khawaga and Abdelalim Stem Cell Research & Therapy (2020) 11:437 Page 10 of 33
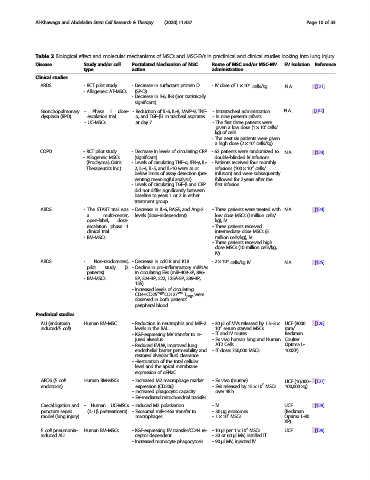
Table 2 Biological effect and molecular mechanisms of MSCs and MSC-EVs in preclinical and clinical studies looking into lung injury
Disease Study and/or cell Postulated Mechanism of MSC Route of MSC and/or MSC-MV EV isolation Reference
type action administration
Clinical studies
6
ARDS - RCT pilot study - Decrease in surfactant protein D - IV dose of 1 × 10 cells/kg N.A. [121]
- Allogeneic AT-MSCs (SP-D)
- Decrease in Il-6, Il-8 (not statistically
significant)
Bronchopulmonary - Phase I dose- - Reduction of IL-6, IL-8, MMP-9, TNF- - Intratracheal administration N.A. [122]
dysplasia (BPD) escalation trial α, and TGF-β1 in tracheal aspirates - In nine preterm infants.
- UC-MSCs at day 7 - The first three patients were
7
given a low dose (1 × 10 cells/
kg) of cells
- The next six patients were given
7
a high dose (2 × 10 cells/kg)
COPD - RCT pilot study - Decrease in levels of circulating CRP - 62 patients were randomized to N.A. [123]
- Allogeneic MSCs (significant) double-blinded IV infusions
(Prochymal; Osiris - Levels of circulating TNF-α, IFN-γ, IL- - Patients received four monthly
6
Therapeutics Inc.) 2, IL-4, IL-5, and IL-10 were at or infusions (100 × 10 cells/
below limits of assay detection (pre- infusion) and were subsequently
venting meaningful analysis) followed for 2 years after the
- Levels of circulating TGF-β and CRP first infusion
did not differ significantly between
baseline to years 1 or 2 in either
treatment group
ARDS - The START trial was - Decrease in IL-6, RAGE, and Ang-2 - Three patients were treated with N.A. [124]
a multi-center, levels (dose-independent) low dose MSCs (1million cells/
open-label, dose- kg), IV
escalation phase 1 - Three patients received
clinical trial intermediate dose MSCs (5
- BM-MSCs million cells/kg), IV
- Three patients received high
dose MSCs (10 million cells/kg,
IV)
6
ARDS - Non-randomized, - Decrease in ccK18 and K18 -2 × 10 cells/kg IV N.A. [125]
pilot study (2 - Decline in pro-inflammatory miRNAs
patients) in circulating EVs (miR-409-3P, 886-
- BM-MSCs 5P, 324-3P, 222, 125A-5P, 339-3P,
155)
- Increased levels of circulating
CD4+CD25 high CD127 low T Regs were
observed in both patients’
peripheral blood
Preclinical studies
ALI (endotoxin Human BM-MSC - Reduction in neutrophils and MIP-2 -30 μl of MVs released by 1.5–3× UCF (3000 [126]
6
induced/E. coli) levels in the BAL 10 serum starved MSCs rpm/
- KGF-expressing MV transfer to in- - IT and IV routes Beckman
jured alveolus - Ex vivo human lung and Human Coulter
- Reduced EVLW, improved lung AT2 Cells. Optima L-
endothelial barrier permeability and - IT dose: 750,000 MSCs 100XP)
restored alveolar fluid clearance
- -Restoration of the total cellular
level and the apical membrane
expression of αENaC
ARDS (E. coli Human BM-MSCs - Increased M2 macrophage marker - Ex vivo (murine) UCF (10,000– [127]
6
endotoxin) expression (CD206) - EVs released by 15 × 10 MSCs 100,000 xg)
- increased phagocytic capacity over 48 h
- EV-mediated mitochondrial transfer
Caecal ligation and - Human UC-MSCs - Induced M2 polarization -IV UCF [128]
puncture sepsis (IL-1β pretreatment) - Exosomal miR-146a transfer to -30 μg exosomes (Beckman
6
model (lung injury) macrophages -1 × 10 MSCs Optima L-80
XP)
6
E. coli pneumonia- Human BM-MSCs - KGF-expressing EV transfer/CD44 re- -10 μl per 1 × 10 MSCs UCF [129]
induced ALI ceptor dependent -30or60 μl MV, instilled IT
- Increased monocyte phagocytosis -90 μl MV, injected IV