Page 25 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 25
Al-Khawaga and Abdelalim Stem Cell Research & Therapy (2020) 11:437 Page 6 of 33
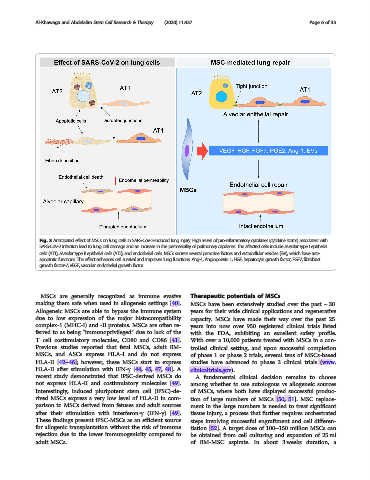
Fig. 3 Anticipated effect of MSCs on lung cells in SARS-CoV-2-induced lung injury. High levels of pro-inflammatory cytokines (cytokine storm) associated with
SARS-CoV-2 infection lead to lung cell damage and an increase in the permeability of pulmonary capillaries. The affected cells include alveolar type I epithelial
cells (AT1), alveolar type II epithelial cells (AT2), and endothelial cells. MSCs secrete several paracrine factors and extracellular vesicles (EVs), which have anti-
apoptotic functions. This effect enhances cell survival and improves lung functions. Ang-1, Angiopoietin 1; HGF, hepatocyte growth factor; FGF7, fibroblast
growth factor-7; VEGF, vascular endothelial growth factor
MSCs are generally recognized as immune evasive Therapeutic potentials of MSCs
making them safe when used in allogeneic settings [40]. MSCs have been extensively studied over the past ~ 30
Allogeneic MSCs are able to bypass the immune system years for their wide clinical applications and regenerative
due to low expression of the major histocompatibility capacity. MSCs have made their way over the past 25
complex-1 (MHC-I) and -II proteins. MSCs are often re- years into now over 950 registered clinical trials listed
ferred to as being “immunoprivileged” due to lack of the with the FDA, exhibiting an excellent safety profile.
T cell costimulatory molecules, CD80 and CD86 [41]. With over a 10,000 patients treated with MSCs in a con-
Previous studies reported that fetal MSCs, adult BM- trolled clinical setting, and upon successful completion
MSCs, and ASCs express HLA-I and do not express of phase 1 or phase 2 trials, several tens of MSCs-based
HLA-II [42–46]; however, these MSCs start to express studies have advanced to phase 3 clinical trials (www.
HLA-II after stimulation with IFN-γ [44, 45, 47, 48]. A clinicaltrials.gov).
recent study demonstrated that iPSC-derived MSCs do A fundamental clinical decision remains to choose
not express HLA-II and costimulatory molecules [49]. among whether to use autologous vs allogeneic sources
Interestingly, induced pluripotent stem cell (iPSC)-de- of MSCs, where both have displayed successful produc-
rived MSCs express a very low level of HLA-II in com- tion of large numbers of MSCs [50, 51]. MSC replace-
parison to MSCs derived from fetuses and adult sources ment in the large numbers is needed to treat significant
after their stimulation with interferon-γ (IFN-γ)[49]. tissue injury, a process that further requires orchestrated
These findings present iPSC-MSCs as an efficient source steps involving successful engraftment and cell differen-
for allogenic transplantation without the risk of immune tiation [52]. A target dose of 100–150 million MSCs can
rejection due to the lower immunogenicity compared to be obtained from cell culturing and expansion of 25 ml
adult MSCs. of BM-MSC aspirate. In about 3 weeks duration, a