Page 34 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 34
Al-Khawaga and Abdelalim Stem Cell Research & Therapy (2020) 11:437 Page 15 of 33
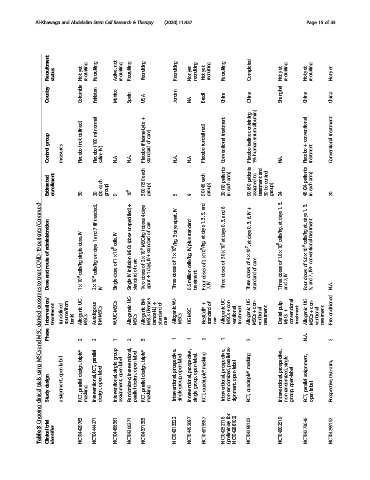
Recruitment status yet Not recruiting Recruiting not Active, recruiting Recruiting Recruiting Recruiting yet Not recruiting yet Not recruiting Recruiting Completed yet Not recruiting yet Not recruiting yet Not
Country Colombia Pakistan Mexico Spain USA Jordan NA Brazil China China Shanghai China China
defined) normal + (Plasma-Lyte treatment containing albumin) conventional treatment
group (not (100 ml care) of (undefined) (saline serum +
Control measures Placebo Placebo IV) saline N.A. N.A. Placebo standard N.A. N.A Placebo Conventional Placebo human 1% N.A. Placebo treatment Conventional
Estimated enrollment 30 20 each (20 group) 9 10 6 each (150 300 group) 5 9 each (45 90 group) patients (10 20 arm) each in patients (60 90 to assigned and treatment control to 30 group) 24 patients (24 48 arm) each in 20
(Continued) needed), (if + unspecified) 4 days (doses care IV apart, and 5, 3, 1, 6 and 3, 0, + IV 3, 1, days at 3, 1, days
patients administration IV dose, 7 and 1 IV cells, (dose MSC/kg of standard 3 days standard plus days at days at 4 × 10 7 ,atdays0,3,6, cells/kg, at, cells/kg treatment
COVID-19 of route single days on 1 × 10 8 of MSCs infusion care 2 × 10 6 of IV+ 1 × 10 6 /kg, of IV, cells/kg, 2 × 10 6 /kg, of 3.0 × 10 7 of of care 1.0 × 10 6 of 5.0 × 10 6 of conventional
treat and cells/kg cells/kg Single-dose IV of doses ± 1 day), doses million doses doses doses of doses IV doses IV+ 7,
to Dose 1×10 6 2×10 6 IV Single standard Two apart Three 0.5 treatment Four IV 7, Three Three standard Three 7, and Four and 5, N.A.
exosomes Intervention/ UC- UC- BM- (Remes- + of WJ- + of UC- con- + UC- con- + pulp + UC- con- + Preconditioned
MSC-derived Phase treatment (undefined source/from bank) Allogenic MSCs Autologous BM-MSCs WJUC-MSCs Allogeneic MSCs Allogenic MSCs temcel-L) standard care Allogenic MSCs UC-MSC NestCell® standard care Allogenic MSCs ventional treatment Allogenic MSCs ventional treatment Dental MSCs conventional treatment Allogenic MSCs ventional treatment
and 2 2 1 2 3 1 1 2 1 as- 2 1 N.A. 2
MSCs label triple* parallel group label interventional, label triple* prospective, prospective, masking prospective, parallel masking prospective, single
using open design, RCT, label single open open design, open-label. open-label. open-label assignment, two-arm,
trials design parallel Interventional, open Interventional, design, parallel Interventional, group, Interventional, group, quadruple* Interventional, non-randomized, quadruple* Interventional, non-randomized, open-label parallel label
clinical Study assignment, RCT, masking design, assignment, Randomized, parallel RCT, masking single single RCT, signment, RCT, group, RCT, open Prospective,
Ongoing for
3 trial NCT04429763 NCT04444271 NCT04456361 NCT04366271 NCT04371393 NCT04313322 NCT04452097 NCT04315987 NCT04252118 (preliminary NCT04288102) NCT04288102 NCT04302519 NCT04273646 NCT04299152
Table Clinical identifier