Page 37 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 37
Al-Khawaga and Abdelalim Stem Cell Research & Therapy (2020) 11:437 Page 18 of 33
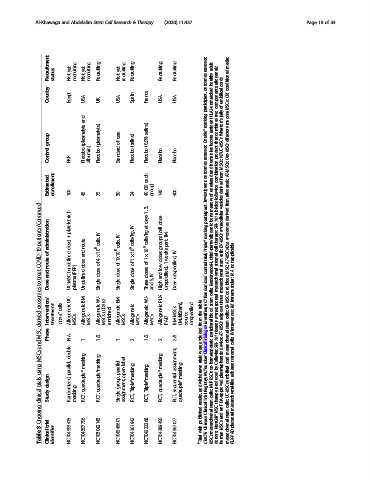
Recruitment status yet Not recruiting yet Not recruiting Recruiting yet Not recruiting Recruiting Recruiting Recruiting assessor; adult healthy allogeneic media; conditioned
Country Egypt USA UK USA Spain France USA USA outcomes participant, (HLA)-unmatched components: cords umbilical of CM MSCs;
and saline) masking antigen two combines jelly Wharton’s mucosa
group (plasmalyte (plasmalyte) care of (saline) (0.9% Double* leukocyte that WJUC-MSCs olfactory
Control PRP. Placebo albumin) Placebo Standard Placebo Placebo Placebo Placebo assessor; human product Om-MSCs
outcomes and combination MSCs; from AT-MSCs;
Estimated enrollment 100 45 75 30 24 each (20 40 group) 140 400 investigator, unrelated of biologic/device derived allogeneic
(Continued) rich 3, 1, days dose participant, marrow bone a SBI-101 vesicles extracellular from derived
patients administration platelet + dose) route IV cells, IV cells, IV cells/kg, at cells/kg (cell groups IM apart, masking Triple* third-party/allogenic therapy; EV-MSCs exosomes
COVID-19 of route (undefined and dose 4 × 10 8 of 1X10 8 of 1 × 10 6 of 1 × 10 6 of doses 1 week IV trial; cell stromal cells; stem AT-MSCs-Exo applicable not
treat and (PRP) dose dose dose doses IV low and unspecified), unspecified, control Remestemcel-L MSC; N.A.
to Dose UC-MSC plasma Undefined Single Single Single Three 5, and High Dose Randomized mesenchymal mesenchymal blood cord intramuscular;
exosomes Intervention/ UC- BM- WJ- (CD362 BM- WJ- PLX- table RCT cells; stem extracorporeal tissue CB-MSC IM
MSC-derived Phase treatment CD34 + cells Allogeneic MSCs Allogeneic MSCs Allogeneic MSCs enriched) Allogeneic MSCs Allogeneic MSCs Allogeneic MSCs Allogeneic PAD BM-MSCs (MultiStem), source unspecified the from identifier; blood peripheral therapy adipose AT-MSCs cells; stem intravenous; IV
and N.A. 1 1/2 1 2 1/2 2 2/3 excluded were ClinicalTrials.gov SBI-101 device; mesenchymal cells;
MSCs double masking masking assignment, trials non-hematopoietic Cellavita; cord stromal
using parallel, quadruple*masking parallel open-label masking withdrawn NCTnumber by plasmapheresis umbilical adherent
trials design quadruple* group, Triple*masking Triple*masking quadruple* sequential Registry; NHPBSC produced
clinical Study Randomized, masking RCT, RCT, Single assignment, RCT, RCT, RCT, RCT, quadruple* and results, Trial cells; stem therapy FDA-approved UC-MSCs cells; mesenchymal-like
Ongoing published Clinical MSC an and stem placental
3 trial NCT04393415 NCT04397796 NCT03042143 NCT04345601 NCT04361942 NCT04333368 NCT04389450 NCT04367077 with Chinese mesenchymal NestCell® MSCs mesenchymal
Table Clinical identifier # Trial ChiCTR MSCs donors; human PLX-PAD