Page 14 - EXOSOMES
P. 14
www.advancedsciencenews.com www.small-journal.com
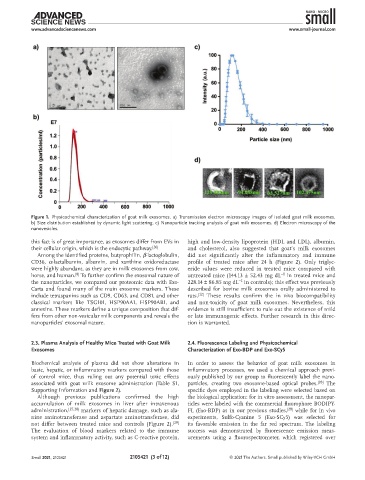
Figure 1. Physicochemical characterization of goat milk exosomes. a) Transmission electron microscopy images of isolated goat milk exosomes.
b) Size distribution established by dynamic light scattering. c) Nanoparticle tracking analysis of goat milk exosomes. d) Electron microscopy of the
nanovesicles.
this fact is of great importance, as exosomes differ from EVs in high and low-density lipoprotein (HDL and LDL), albumin,
their cellular origin, which is the endocytic pathway. [26] and cholesterol, also suggested that goat’s milk exosomes
Among the identified proteins, butyrophilin, β-lactoglobulin, did not significantly alter the inflammatory and immune
CD36, α-lactalbumin, albumin, and xanthine oxidoreductase profile of treated mice after 24 h (Figure 2). Only triglyc-
were highly abundant, as they are in milk exosomes from cow, eride values were reduced in treated mice compared with
[8]
−1
horse, and human. To further confirm the exosomal nature of untreated mice (144.13 ± 52.43 mg dL in treated mice and
the nanoparticles, we compared our proteomic data with Exo- 228.14 ± 86.85 mg dL in controls); this effect was previously
−1
Carta and found many of the main exosome markers. These described for bovine milk exosomes orally administered to
include tetraspanins such as CD9, CD63, and CD81, and other rats. [12] These results confirm the in vivo biocompatibility
classical markers like TSG101, HSP90AA1, HSP90AB1, and and non-toxicity of goat milk exosomes. Nevertheless, this
annexins. These markers define a unique composition that dif- evidence is still insufficient to rule out the existence of mild
fers from other non-vesicular milk components and reveals the or late immunogenic effects. Further research in this direc-
nanoparticles’ exosomal nature. tion is warranted.
2.3. Plasma Analysis of Healthy Mice Treated with Goat Milk 2.4. Fluorescence Labeling and Physicochemical
Exosomes Characterization of Exo-BDP and Exo-SCy5
Biochemical analysis of plasma did not show alterations in In order to assess the behavior of goat milk exosomes in
basic, hepatic, or inflammatory markers compared with those inflammatory processes, we used a chemical approach previ-
of control mice, thus ruling out any potential toxic effects ously published by our group to fluorescently label the nano-
associated with goat milk exosome administration (Table S1, particles, creating two exosome-based optical probes. [28] The
Supporting Information and Figure 2). specific dyes employed in the labeling were selected based on
Although previous publications confirmed the high the biological application: for in vitro assessment, the nanopar-
accumulation of milk exosomes in liver after intravenous ticles were labeled with the commercial fluorophore BODIPY-
administration, [27,28] markers of hepatic damage, such as ala- FL (Exo-BDP) as in our previous studies, [28] while for in vivo
nine aminotransferase and aspartate aminotransferase, did experiments, Sulfo-Cyanine 5 (Exo-SCy5) was selected for
not differ between treated mice and controls (Figure 2). [29] its favorable emission in the far red spectrum. The labeling
The evaluation of blood markers related to the immune success was demonstrated by fluorescence emission meas-
system and inflammatory activity, such as C-reactive protein, urements using a fluorospectrometer, which registered over
Small 2021, 2105421 2105421 (3 of 12) © 2021 The Authors. Small published by Wiley-VCH GmbH