Page 25 - Risk Reduction Series - Documentation Essentials (Part One)
P. 25
SVMIC Risk Reduction Series: Documentation Essentials
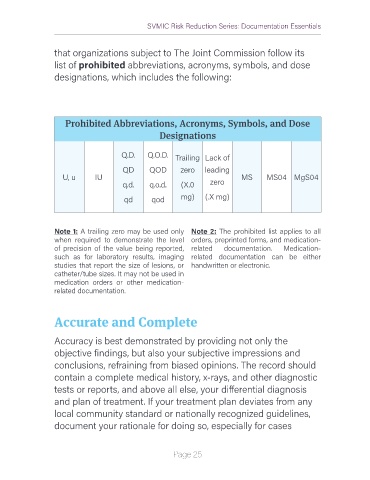
that organizations subject to The Joint Commission follow its
list of prohibited abbreviations, acronyms, symbols, and dose
designations, which includes the following:
Prohibited Abbreviations, Acronyms, Symbols, and Dose
Designations
Q.D. Q.O.D. Trailing Lack of
QD QOD zero leading
U, u IU MS MS04 MgS04
q.d. q.o.d. (X.0 zero
qd qod mg) (.X mg)
Note 1: A trailing zero may be used only Note 2: The prohibited list applies to all
when required to demonstrate the level orders, preprinted forms, and medication-
of precision of the value being reported, related documentation. Medication-
such as for laboratory results, imaging related documentation can be either
studies that report the size of lesions, or handwritten or electronic.
catheter/tube sizes. It may not be used in
medication orders or other medication-
related documentation.
Accurate and Complete
Accuracy is best demonstrated by providing not only the
objective findings, but also your subjective impressions and
conclusions, refraining from biased opinions. The record should
contain a complete medical history, x-rays, and other diagnostic
tests or reports, and above all else, your differential diagnosis
and plan of treatment. If your treatment plan deviates from any
local community standard or nationally recognized guidelines,
document your rationale for doing so, especially for cases
Page 25