Page 7 - E-LKM Asam Basa_Neat
P. 7
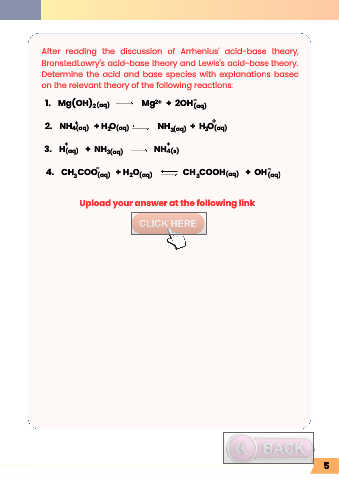
After reading the discussion of Arrhenius' acid-base theory,
BronstedLowry's acid-base theory and Lewis's acid-base theory.
Determine the acid and base species with explanations based
on the relevant theory of the following reactions:
-
2+
(aq)
1. Mg(OH) Mg + 2OH (aq)
2
+ +
4
(aq)
2. NH + H O NH + H O (aq)
2 (aq)
3
3 (aq)
+ +
3. H + NH NH
4 (s)
(aq)
3 (aq)
- -
(aq)
(aq)
4. CH COO + H O CH COOH + OH (aq)
(aq)
2
3
3
Upload your answer at the following link
5