Page 12 - E-LKM Asam Basa_Neat
P. 12
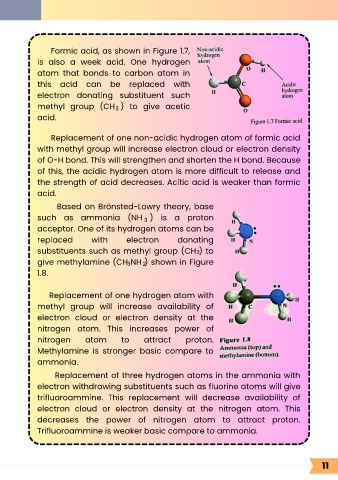
Formic acid, as shown in Figure 1.7,
is also a week acid. One hydrogen
atom that bonds to carbon atom in
this acid can be replaced with
electron donating substituent such
methyl group (CH ) to give acetic
3
acid.
Replacement of one non-acidic hydrogen atom of formic acid
with methyl group will increase electron cloud or electron density
of O-H bond. This will strengthen and shorten the H bond. Because
of this, the acidic hydrogen atom is more difficult to release and
the strength of acid decreases. Acitic acid is weaker than formic
acid.
Based on Brönsted-Lowry theory, base
such as ammonia (NH ) is a proton
3
acceptor. One of its hydrogen atoms can be
replaced with electron donating
substituents such as methyl group (CH ) to
3
give methylamine (CH NH ) shown in Figure
2
3
1.8.
Replacement of one hydrogen atom with
methyl group will increase availability of
electron cloud or electron density at the
nitrogen atom. This increases power of
nitrogen atom to attract proton.
Methylamine is stronger basic compare to
ammonia.
Replacement of three hydrogen atoms in the ammonia with
electron withdrawing substituents such as fluorine atoms will give
trifluoroammine. This replacement will decrease availability of
electron cloud or electron density at the nitrogen atom. This
decreases the power of nitrogen atom to attract proton.
Trifluoroammine is weaker basic compare to ammonia.
11