Page 31 - E-LKM ACID BASE
P. 31
Lembar Kerja Mahasiswa - Asam Basa
Exercise 2
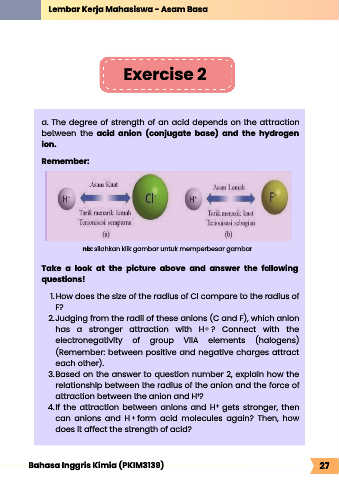
a. The degree of strength of an acid depends on the attraction
between the acid anion (conjugate base) and the hydrogen
ion.
Remember:
nb: silahkan klik gambar untuk memperbesar gambar
Take a look at the picture above and answer the following
questions!
1. How does the size of the radius of Cl compare to the radius of
F?
2. Judging from the radii of these anions (C and F), which anion
has a stronger attraction with H ? Connect with the
+
electronegativity of group VIIA elements (halogens)
(Remember: between positive and negative charges attract
each other).
3. Based on the answer to question number 2, explain how the
relationship between the radius of the anion and the force of
+
attraction between the anion and H ?
+
4. If the attraction between anions and H gets stronger, then
can anions and H form acid molecules again? Then, how
+
does it affect the strength of acid?
Bahasa Inggris Kimia (PKIM3139) 27