Page 28 - 25268
P. 28
2
Cleaning, Disinfection and Sterilization Cleaning, Disinfection and Sterilization
7 Cleaning, Disinfection and Sterilization
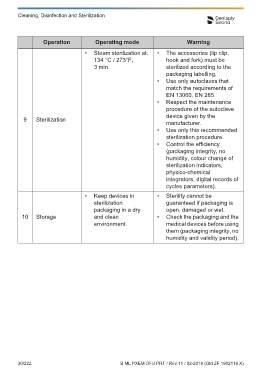
Operation Operating mode Warning
• Steam sterilization at: • The accessories (lip clip, 7.1 General Recommendations
134 °C / 273°F, hook and fork) must be
3 min. sterilized according to the • The device does not contain user serviceable parts. The service
packaging labelling. and repair should be provided by factory trained service personnel
• Use only autoclaves that only.
match the requirements of • After each use, all objects that were in contact with infectious
EN 13060, EN 285.
• Respect the maintenance agents should be cleaned using towels impregnated with a
procedure of the autoclave disinfecting and detergent solution (a bactericidal, fungicidal and
device given by the aldehyde free solution). Use of chemical agents may cause
9 Sterilization
manufacturer. damage to the equipment. We recommend to use only a
• Use only this recommended disinfecting solution which is approved for its efficacy
sterilization procedure. (VAH/DGHM-listing, CE marking, FDA approval).
• Control the efficiency • The lip clip and the hook must be sterilized between treatments.
(packaging integrity, no Please note that the measuring cable cannot be autoclaved.
humidity, colour change of • In addition, a fork is not included in the Propex Pixi™ but it can be
sterilization indicators,
physico-chemical used and should follow the same procedure as the lip clip and hook.
integrators, digital records of • Follow the “Disinfection and sterilization procedure” described in
cycles parameters). section 7.2.
• Keep devices in • Sterility cannot be • The user is responsible for the sterility of the lip clip, the connection
sterilization guaranteed if packaging is hook and the fork for the first cycle and each further usage.
packaging in a dry open, damaged or wet. • All damaged accessories should be discarded and dirty
10 Storage and clean • Check the packaging and the accessories should be cleaned and sterilized per the procedure
environment. medical devices before using described in section 7.2.
them (packaging integrity, no
humidity and validity period).
30/222 # B ML PXEM DFU PRT / Rev.11 / 02-2018 (Old ZF 1902118.X) 25268-EYAL - 25268_pnim_eyal | 1 - A | 18-05-15 | 13:58:38 | SR:-- | Yellow 25268-EYAL - 25268_pnim_eyal | 1 - A | 18-05-15 | 13:58:38 | SR:-- | Cyan 25268-EYAL - 25268_pnim_eyal | 1 - A | 18-05-15 | 13:58:38 | SR:-- | Magenta #25
1