Page 3 - Group 4_Project_CESP2021
P. 3
PRELIMINARY
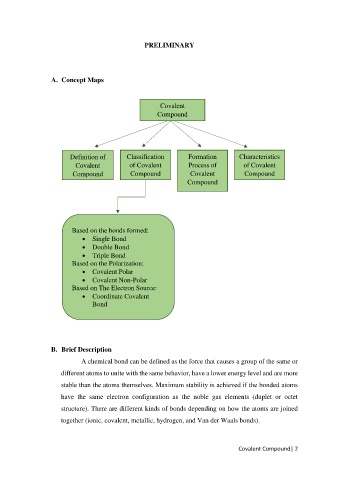
A. Concept Maps
Covalent
Compound
Definition of Classification Formation Characteristics
Covalent of Covalent Process of of Covalent
Compound Compound Covalent Compound
Compound
Based on the bonds formed:
• Single Bond
• Double Bond
• Triple Bond
Based on the Polarization:
• Covalent Polar
• Covalent Non-Polar
Based on The Electron Source:
• Coordinate Covalent
Bond
B. Brief Description
A chemical bond can be defined as the force that causes a group of the same or
different atoms to unite with the same behavior, have a lower energy level and are more
stable than the atoms themselves. Maximum stability is achieved if the bonded atoms
have the same electron configuration as the noble gas elements (duplet or octet
structure). There are different kinds of bonds depending on how the atoms are joined
together (ionic, covalent, metallic, hydrogen, and Van der Waals bonds).
Covalent Compound| 2