Page 282 - Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice
P. 282
Metabolic Acid-Base Disorders 273
8.00 10
7.90
7.80
PaCO 2 Fixed at 40 mm Hg
7.70 20
pH 7.60 [H + ] (nEq/L)
– = 0.7 30
Mean titration curve with Δ PaCO 2 /Δ HCO 3
7.50
7.40 40
24 36 48 60
–
Plasma [HCO 3 ] (mEq/L)
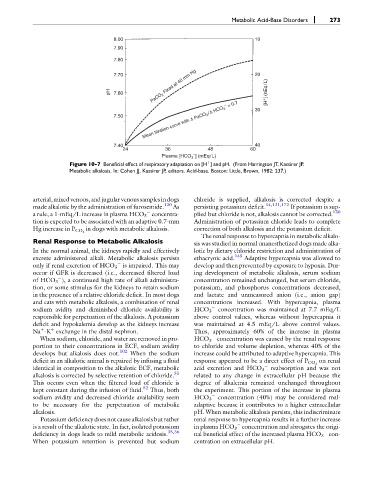
Figure 10-7 Beneficial effect of respiratory adaptation on [H ] and pH. (From Harrington JT, Kassirer JP.
þ
Metabolic alkalosis. In: Cohen JJ, Kassirer JP, editors. Acid-base. Boston: Little, Brown, 1982: 237.)
arterial, mixedvenous,andjugular venous samples in dogs chloride is supplied, alkalosis is corrected despite a
made alkalotic by the administration of furosemide. 120 As persisting potassium deficit. 14,131,172 If potassium is sup-
a rule, a 1-mEq/L increase in plasma HCO 3 concentra- plied but chloride is not, alkalosis cannot be corrected. 130
tion is expected to be associated with an adaptive 0.7-mm Administration of potassium chloride leads to complete
in dogs with metabolic alkalosis. correction of both alkalosis and the potassium deficit.
Hg increase in P CO 2
The renal response to hypercapnia in metabolic alkalo-
Renal Response to Metabolic Alkalosis sis was studied in normal unanesthetized dogs made alka-
In the normal animal, the kidneys rapidly and effectively lotic by dietary chloride restriction and administration of
excrete administered alkali. Metabolic alkalosis persists ethacrynic acid. 145 Adaptive hypercapnia was allowed to
only if renal excretion of HCO 3 is impaired. This may develop and then prevented by exposure to hypoxia. Dur-
occur if GFR is decreased (i.e., decreased filtered load ing development of metabolic alkalosis, serum sodium
of HCO 3 ), a continued high rate of alkali administra- concentration remained unchanged, but serum chloride,
tion, or some stimulus for the kidneys to retain sodium potassium, and phosphorus concentrations decreased,
in the presence of a relative chloride deficit. In most dogs and lactate and unmeasured anion (i.e., anion gap)
and cats with metabolic alkalosis, a combination of renal concentrations increased. With hypercapnia, plasma
sodium avidity and diminished chloride availability is HCO 3 concentration was maintained at 7.7 mEq/L
responsible for perpetuation of the alkalosis. A potassium above control values, whereas without hypercapnia it
deficit and hypokalemia develop as the kidneys increase was maintained at 4.5 mEq/L above control values.
þ
þ
Na -K exchange in the distal nephron. Thus, approximately 60% of the increase in plasma
When sodium, chloride, and water are removed in pro- HCO 3 concentration was caused by the renal response
portion to their concentrations in ECF, sodium avidity to chloride and volume depletion, whereas 40% of the
develops but alkalosis does not. 102 When the sodium increase could be attributed to adaptive hypercapnia. This
deficit in an alkalotic animal is repaired by infusing a fluid response appeared to be a direct effect of P CO 2 on renal
identical in composition to the alkalotic ECF, metabolic acid excretion and HCO 3 reabsorption and was not
alkalosis is corrected by selective retention of chloride. 51 related to any change in extracellular pH because the
This occurs even when the filtered load of chloride is degree of alkalemia remained unchanged throughout
kept constant during the infusion of fluid. 52 Thus, both the experiment. This portion of the increase in plasma
sodium avidity and decreased chloride availability seem HCO 3 concentration (40%) may be considered mal-
to be necessary for the perpetuation of metabolic adaptive because it contributes to a higher extracellular
alkalosis. pH. When metabolic alkalosis persists, this indiscriminate
Potassium deficiency does not cause alkalosis but rather renal response to hypercapnia results in a further increase
is a result of the alkalotic state. In fact, isolated potassium in plasma HCO 3 concentration and abrogates the origi-
deficiency in dogs leads to mild metabolic acidosis. 35,36 nal beneficial effect of the increased plasma HCO 3 con-
When potassium retention is prevented but sodium centration on extracellular pH.