Page 634 - The Toxicology of Fishes
P. 634
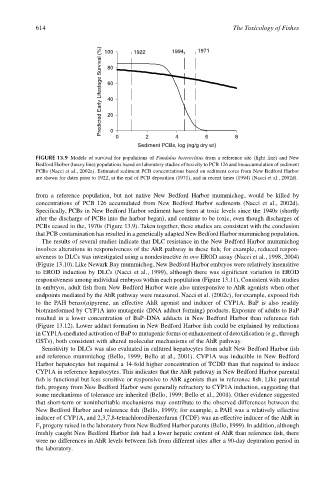
614 100 1922 1994 1971 The Toxicology of Fishes
Predicted Early Lifestage Survival (%) 80
60
40
20
0
0 2 4 6 8
Sediment PCBs, log (ng/g dry wt)
FIGURE 13.9 Models of survival for populations of Fundulus heteroclitus from a reference site (light line) and New
Bedford Harbor (heavy line) populations based on laboratory studies of toxicity to PCB 126 and bioaccumulation of sediment
PCBs (Nacci et al., 2002a). Estimated sediment PCB concentrations based on sediment cores from New Bedford Harbor
are shown for dates prior to 1922, at the end of PCB deposition (1971), and in recent times (1994) (Nacci et al., 2002d).
from a reference population, but not native New Bedford Harbor mummichog, would be killed by
concentrations of PCB 126 accumulated from New Bedford Harbor sediments (Nacci et al., 2002d).
Specifically, PCBs in New Bedford Harbor sediment have been at toxic levels since the 1940s (shortly
after the discharge of PCBs into the harbor began), and continue to be toxic, even though discharges of
PCBs ceased in the, 1970s (Figure 13.9). Taken together, these studies are consistent with the conclusion
that PCB contamination has resulted in a genetically adapted New Bedford Harbor mummichog population.
The results of several studies indicate that DLC resistance in the New Bedford Harbor mummichog
involves alterations in responsiveness of the AhR pathway in these fish; for example, reduced respon-
siveness to DLCs was investigated using a nondestructive in ovo EROD assay (Nacci et al., 1998, 2004)
(Figure 13.10). Like Newark Bay mummichog, New Bedford Harbor embryos were relatively insensitive
to EROD induction by DLCs (Nacci et al., 1999), although there was significant variation in EROD
responsiveness among individual embryos within each population (Figure 13.11). Consistent with studies
in embryos, adult fish from New Bedford Harbor were also unresponsive to AhR agonists when other
endpoints mediated by the AhR pathway were measured. Nacci et al. (2002c), for example, exposed fish
to the PAH benzo(a)pyrene, an effective AhR agonist and inducer of CYP1A. BaP is also readily
biotransformed by CYP1A into mutagenic (DNA adduct forming) products. Exposure of adults to BaP
resulted in a lower concentration of BaP–DNA adducts in New Bedford Harbor than reference fish
(Figure 13.12). Lower adduct formation in New Bedford Harbor fish could be explained by reductions
in CYP1A-mediated activation of BaP to mutagenic forms or enhancement of detoxification (e.g., through
GSTs), both consistent with altered molecular mechanisms of the AhR pathway.
Sensitivity to DLCs was also evaluated in cultured hepatocytes from adult New Bedford Harbor fish
and reference mummichog (Bello, 1999; Bello at al., 2001). CYP1A was inducible in New Bedford
Harbor hepatocytes but required a 14-fold higher concentration of TCDD than that required to induce
CYP1A in reference hepatocytes. This indicates that the AhR pathway in New Bedford Harbor parental
fish is functional but less sensitive or responsive to AhR agonists than in reference fish. Like parental
fish, progeny from New Bedford Harbor were generally refractory to CYP1A induction, suggesting that
some mechanisms of tolerance are inherited (Bello, 1999; Bello et al., 2001). Other evidence suggested
that short-term or noninheritable mechanisms may contribute to the observed differences between the
New Bedford Harbor and reference fish (Bello, 1999); for example, a PAH was a relatively effective
inducer of CYP1A, and 2,3,7,8-tetrachlorodibenzofuran (TCDF) was an effective inducer of the AhR in
F progeny raised in the laboratory from New Bedford Harbor parents (Bello, 1999). In addition, although
1
freshly caught New Bedford Harbor fish had a lower hepatic content of AhR than reference fish, there
were no differences in AhR levels between fish from different sites after a 90-day depuration period in
the laboratory.