Page 637 - The Toxicology of Fishes
P. 637
Toxicity Resistance 617
3
2
2 2
1
1 1
6 6
5 4
5
3 3
2
2
1 1
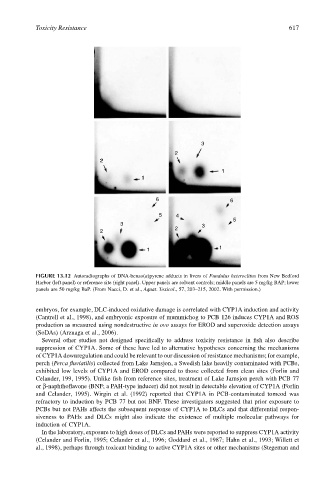
FIGURE 13.12 Autoradiographs of DNA-benzo(a)pyrene adducts in livers of Fundulus heteroclitus from New Bedford
Harbor (left panel) or reference site (right panel). Upper panels are solvent controls; middle panels are 5 mg/kg BAP; lower
panels are 50 mg/kg BaP. (From Nacci, D. et al., Aquat. Toxicol., 57, 203–215, 2002. With permission.)
embryos, for example, DLC-induced oxidative damage is correlated with CYP1A induction and activity
(Cantrell et al., 1998), and embryonic exposure of mummichog to PCB 126 induces CYP1A and ROS
production as measured using nondestructive in ovo assays for EROD and superoxide detection assays
(SoDAs) (Arzuaga et al., 2006).
Several other studies not designed specifically to address toxicity resistance in fish also describe
suppression of CYP1A. Some of these have led to alternative hypotheses concerning the mechanisms
of CYP1A downregulation and could be relevant to our discussion of resistance mechanisms; for example,
perch (Perca fluviatilis) collected from Lake Jarnsjon, a Swedish lake heavily contaminated with PCBs,
exhibited low levels of CYP1A and EROD compared to those collected from clean sites (Forlin and
Celander, 199, 1995). Unlike fish from reference sites, treatment of Lake Jarnsjon perch with PCB 77
or β-naphthoflavone (BNF; a PAH-type inducer) did not result in detectable elevation of CYP1A (Forlin
and Celander, 1995). Wirgin et al. (1992) reported that CYP1A in PCB-contaminated tomcod was
refractory to induction by PCB 77 but not BNF. These investigators suggested that prior exposure to
PCBs but not PAHs affects the subsequent response of CYP1A to DLCs and that differential respon-
siveness to PAHs and DLCs might also indicate the existence of multiple molecular pathways for
induction of CYP1A.
In the laboratory, exposure to high doses of DLCs and PAHs were reported to suppress CYP1A activity
(Celander and Forlin, 1995; Celander et al., 1996; Goddard et al., 1987; Hahn et al., 1993; Willett et
al., 1998), perhaps through toxicant binding to active CYP1A sites or other mechanisms (Stegeman and