Page 1200 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 1200
1132 SECTION | XVII Analytical Toxicology
VetBooks.ir liquid sample, such as urine or serum, or it may be a compounds before eluting the analytes. In this way, SPE
may provide both extraction and cleanup of a sample.
homogenate of a nonliquid sample. When the acceptor
phase is properly chosen, the analytes will partition into
it. Organochlorine pesticides are a good example. Most Derivatization Methods
are not very polar compounds, so if they are contained in
an aqueous solution and that solution is shaken up with There are some poisons that are not amenable to detection
methylene chloride (which is not miscible with water), by any of the above methods. An example of such a
the organochlorines will partition into the less polar meth- compound is ethylene glycol. Its low molecular weight
ylene chloride phase. The methylene chloride will settle and high polarity make it unsuitable for GC analysis. It
out from the water and it can then be removed, subjected lacks a distinctive UV spectrum and does not contain a
to further cleanup procedures, concentrated, or analyzed fluorophore, making it unsuitable for HPLC with UV or
directly. LLE is a very effective technique for extracting fluorescence detection. And it lacks any acidic or basic
many different types of compounds out of a sample sites on the molecule making it unsuitable for LC-MS. In a
case like this, the compound may be chemically altered
into a derivative that is suitable for one of the above meth-
QuEChERS Extraction
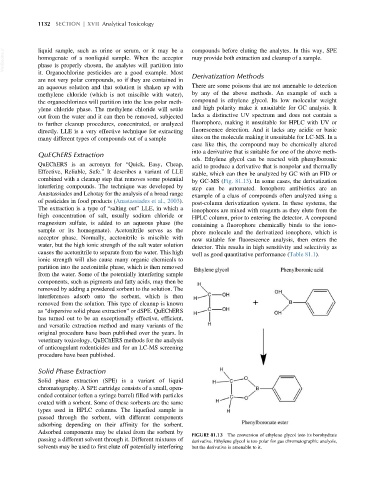
ods. Ethylene glycol can be reacted with phenylboronic
QuEChERS is an acronym for “Quick, Easy, Cheap, acid to produce a derivative that is nonpolar and thermally
Effective, Reliable, Safe.” It describes a variant of LLE stable, which can then be analyzed by GC with an FID or
combined with a cleanup step that removes some potential by GC-MS (Fig. 81.13). In some cases, the derivatization
interfering compounds. The technique was developed by step can be automated. Ionophore antibiotics are an
Anastassiades and Lehotay for the analysis of a broad range example of a class of compounds often analyzed using a
of pesticides in food products (Anastassiades et al., 2003). post-column derivatization system. In these systems, the
The extraction is a type of “salting out” LLE, in which a ionophores are mixed with reagents as they elute from the
high concentration of salt, usually sodium chloride or HPLC column, prior to entering the detector. A compound
magnesium sulfate, is added to an aqueous phase (the containing a fluorophore chemically binds to the iono-
sample or its homogenate). Acetonitrile serves as the phore molecule and the derivatized ionophore, which is
acceptor phase. Normally, acetonitrile is miscible with now suitable for fluorescence analysis, then enters the
water, but the high ionic strength of the salt water solution detector. This results in high sensitivity and selectivity as
causes the acetonitrile to separate from the water. This high well as good quantitative performance (Table 81.1).
ionic strength will also cause many organic chemicals to
partition into the acetonitrile phase, which is then removed
from the water. Some of the potentially interfering sample
components, such as pigments and fatty acids, may then be
removed by adding a powdered sorbent to the solution. The
interferences adsorb onto the sorbent, which is then
removed from the solution. This type of cleanup is known
as “dispersive solid phase extraction” or dSPE. QuEChERS
has turned out to be an exceptionally effective, efficient,
and versatile extraction method and many variants of the
original procedure have been published over the years. In
veterinary toxicology, QuEChERS methods for the analysis
of anticoagulant rodenticides and for an LC-MS screening
procedure have been published.
Solid Phase Extraction
Solid phase extraction (SPE) is a variant of liquid
chromatography. A SPE cartridge consists of a small, open-
ended container (often a syringe barrel) filled with particles
coated with a sorbent. Some of these sorbents are the same
types used in HPLC columns. The liquefied sample is
passed through the sorbent, with different components
adsorbing depending on their affinity for the sorbent.
Adsorbed components may be eluted from the sorbent by
FIGURE 81.13 The conversion of ethylene glycol into its borohydrate
passing a different solvent through it. Different mixtures of derivative. Ethylene glycol is too polar for gas chromatographic analysis,
solvents may be used to first elute off potentially interfering but the derivative is amenable to it.