Page 1199 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 1199
Analytical Toxicology and Sample Submission Requirements Chapter | 81 1131
VetBooks.ir
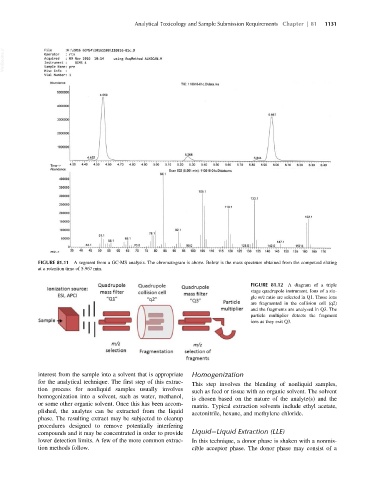
FIGURE 81.11 A segment from a GC-MS analysis. The chromatogram is above. Below is the mass spectrum obtained from the compound eluting
at a retention time of 5.967 min.
FIGURE 81.12 A diagram of a triple
stage quadrupole instrument. Ions of a sin-
gle m/z ratio are selected in Q1. Those ions
are fragmented in the collision cell (q2)
and the fragments are analyzed in Q3. The
particle multiplier detects the fragment
ions as they exit Q3.
interest from the sample into a solvent that is appropriate Homogenization
for the analytical technique. The first step of this extrac-
This step involves the blending of nonliquid samples,
tion process for nonliquid samples usually involves such as feed or tissue with an organic solvent. The solvent
homogenization into a solvent, such as water, methanol, is chosen based on the nature of the analyte(s) and the
or some other organic solvent. Once this has been accom- matrix. Typical extraction solvents include ethyl acetate,
plished, the analytes can be extracted from the liquid acetonitrile, hexane, and methylene chloride.
phase. The resulting extract may be subjected to cleanup
procedures designed to remove potentially interfering
compounds and it may be concentrated in order to provide Liquid Liquid Extraction (LLE)
lower detection limits. A few of the more common extrac- In this technique, a donor phase is shaken with a nonmis-
tion methods follow. cible acceptor phase. The donor phase may consist of a