Page 187 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 187
154 SECTION | I General
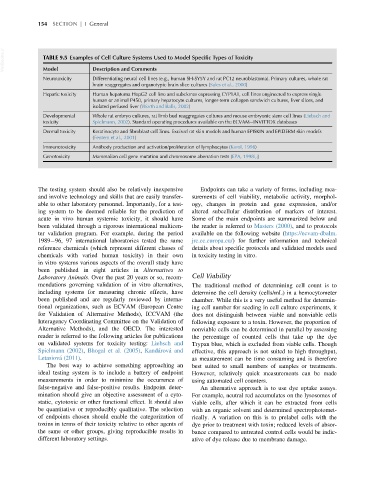
VetBooks.ir TABLE 9.5 Examples of Cell Culture Systems Used to Model Specific Types of Toxicity
Model
Description and Comments
Neurotoxicity Differentiating neural cell lines (e.g., human SH-SY5Y and rat PC12 neuroblastoma). Primary cultures, whole rat
brain reaggregates and organotypic brain slice cultures (Sales et al., 2000)
Hepatic toxicity Human hepatoma HepG2 cell line and subclones expressing CYP1A1, cell lines engineered to express single
human or animal P450, primary hepatocyte cultures, longer-term collagen sandwich cultures, liver slices, and
isolated perfused liver (Worth and Balls, 2002)
Developmental Whole rat embryo cultures, rat limb bud reaggregates cultures and mouse embryonic stem cell lines (Liebsch and
toxicity Spielmann, 2002). Standard operating procedures available on the ECVAM INVITTOX databases
Dermal toxicity Keratinocyte and fibroblast cell lines. Excised rat skin models and human EPISKIN and EPIDERM skin models
(Fentem et al., 2001)
Immunotoxicity Antibody production and activation/proliferation of lymphocytes (Karol, 1998)
Genotoxicity Mammalian cell gene mutation and chromosome aberration tests (EPA, 1998i,j)
The testing system should also be relatively inexpensive Endpoints can take a variety of forms, including mea-
and involve technology and skills that are easily transfer- surements of cell viability, metabolic activity, morphol-
able to other laboratory personnel. Importantly, for a test- ogy, changes in protein and gene expression, and/or
ing system to be deemed reliable for the prediction of altered subcellular distribution of markers of interest.
acute in vivo human systemic toxicity, it should have Some of the main endpoints are summarized below and
been validated through a rigorous international multicen- the reader is referred to Masters (2000), and to protocols
ter validation program. For example, during the period available on the following website (https://ecvam-dbalm.
1989 96, 97 international laboratories tested the same jrc.ec.europa.eu/) for further information and technical
reference chemicals (which represent different classes of details about specific protocols and validated models used
chemicals with varied human toxicity) in their own in toxicity testing in vitro.
in vitro systems various aspects of the overall study have
been published in eight articles in Alternatives to
Laboratory Animals. Over the past 20 years or so, recom- Cell Viability
mendations governing validation of in vitro alternatives, The traditional method of determining cell count is to
including systems for measuring chronic effects, have determine the cell density (cells/mL) in a hemocytometer
been published and are regularly reviewed by interna- chamber. While this is a very useful method for determin-
tional organizations, such as ECVAM (European Centre ing cell number for seeding in cell culture experiments, it
for Validation of Alternative Methods), ICCVAM (the does not distinguish between viable and nonviable cells
Interagency Coordinating Committee on the Validation of following exposure to a toxin. However, the proportion of
Alternative Methods), and the OECD. The interested nonviable cells can be determined in parallel by assessing
reader is referred to the following articles for publications the percentage of counted cells that take up the dye
on validated systems for toxicity testing: Liebsch and Trypan blue, which is excluded from viable cells. Though
Spielmann (2002), Bhogal et al. (2005), Kanda ´rova ´ and effective, this approach is not suited to high throughput,
Letasiova ´ (2011). as measurement can be time consuming and is therefore
The best way to achieve something approaching an best suited to small numbers of samples or treatments.
ideal testing system is to include a battery of endpoint However, relatively quick measurements can be made
measurements in order to minimize the occurrence of using automated cell counters.
false-negative and false-positive results. Endpoint deter- An alternative approach is to use dye uptake assays.
mination should give an objective assessment of a cyto- For example, neutral red accumulates on the lysosomes of
static, cytotoxic or other functional effect. It should also viable cells, after which it can be extracted from cells
be quantitative or reproducibly qualitative. The selection with an organic solvent and determined spectrophotomet-
of endpoints chosen should enable the categorization of rically. A variation on this is to prelabel cells with the
toxins in terms of their toxicity relative to other agents of dye prior to treatment with toxin; reduced levels of absor-
the same or other groups, giving reproducible results in bance compared to untreated control cells would be indic-
different laboratory settings. ative of dye release due to membrane damage.