Page 379 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 379
346 SECTION | III Nanoparticles, Radiation and Carcinogens
VetBooks.ir Aralkylation Involves the Addition of an Aralkyl
Group to DNA
An aralkyl (arylalkyl) contains an alkyl-substituted aro-
matic ring (Dipple, 1995; Okey et al., 1998). Aralkylating
carcinogenic agents add an electrophilic aralkyl group to
nucleophilic sites in DNA. Carcinogens that transfer an
aralkyl group to DNA include the polycyclic aromatic
hydrocarbons and related compounds, alkyl benzenes,
pyrrolizidine alkaloids, and nitroaromatics that are acti-
vated through the formation of dihydrodiol epoxide
(Dipple, 1995).
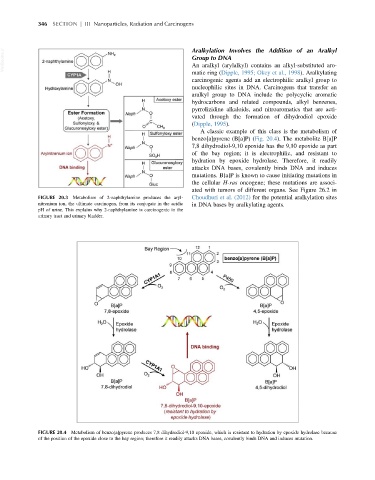
A classic example of this class is the metabolism of
benzo[a]pyrene (B[a]P) (Fig. 20.4). The metabolite B[a]P
7,8 dihydrodiol-9,10 epoxide has the 9,10 epoxide as part
of the bay region; it is electrophilic, and resistant to
hydration by epoxide hydrolase. Therefore, it readily
attacks DNA bases, covalently binds DNA and induces
mutations. B[a]P is known to cause initiating mutations in
the cellular H-ras oncogene; these mutations are associ-
ated with tumors of different organs. See Figure 26.2 in
FIGURE 20.3 Metabolism of 2-naphthylamine produces the aryl- Choudhuri et al. (2012) for the potential aralkylation sites
nitrenium ion, the ultimate carcinogen, from its conjugate in the acidic in DNA bases by aralkylating agents.
pH of urine. This explains why 2-naphthylamine is carcinogenic in the
urinary tract and urinary bladder.
FIGURE 20.4 Metabolism of benzo[a]pyrene produces 7,8 dihydrodiol-9,10 epoxide, which is resistant to hydration by epoxide hydrolase because
of the position of the epoxide close to the bay region; therefore it readily attacks DNA bases, covalently binds DNA and induces mutation.