Page 383 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 383
350 SECTION | III Nanoparticles, Radiation and Carcinogens
VetBooks.ir carry oncogenes derived from cellular proto-oncogenes or identical to that of the c-onc products, and the expres-
sion is generally unregulated.
that are involved in mitogenic signaling and growth
control (Butel, 2000).
The model for the acquisition of oncogenes by retro-
viruses from cellular proto-oncogenes was first provided Radiation Carcinogenesis
by Takeya and Hanafusa (1983) from their work on the c-
The Risk of Radiation-Induced Carcinogenesis
Src proto-oncogene. Cellular proto-oncogenes contain
introns while the corresponding viral oncogenes lack Is Directly Related to the Amount of Energy
introns. The retroviral oncogene capture model postulates Deposited Into the Tissue by Radiation
that the c-onc sequence was captured by virus through In the following discussion, radiation will refer only to
recombination that occurred at the level of proviral DNA. ionizing radiation. A radiation dose to tissue is expressed
Retroviruses replicate inside the cell through a DNA inter- as absorbed energy per unit tissue mass. The Gray (Gy) is
mediate, called provirus, which is integrated into the chro- the unit of radiation dose and is quantified as 1 joule/kg
mosomal DNA of the infected cell. Chance integration of tissue. The older unit rad is still used and 1 rad 5 0.01 Gy.
provirus next to the cellular proto-oncogene creates a Carcinogenic potential of radiation depends upon the
viral-cellular fusion gene. Read-through transcription of absorbed dose (energy).
this fusion gene creates a hybrid (viral 1 cellular) RNA. LET (linear energy transfer; L) is a measure of the
Processing of this read-through transcript removes the rate at which energy (E) is deposited to the absorbing
introns. When this hybrid RNA sequence undergoes medium per unit distance (l) traversed by the radiation
recombination with the viral RNA during reverse- (L 5 dE/dl; if the distance traversed is measured in mm,
transcription, the cellular oncogene (without the introns) is then L 5 keV/mm). Consequently, high-LET radiations
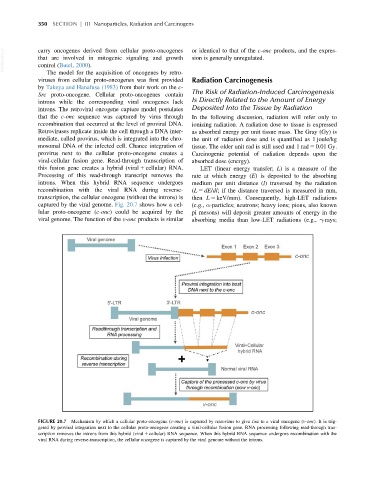
captured by the viral genome. Fig. 20.7 shows how a cel- (e.g., α-particles; neutrons; heavy ions; pions, also known
lular proto-oncogene (c-onc) could be acquired by the pi mesons) will deposit greater amounts of energy in the
viral genome. The function of the v-onc products is similar absorbing media than low-LET radiations (e.g., γ-rays;
FIGURE 20.7 Mechanism by which a cellular proto-oncogene (c-onc) is captured by retrovirus to give rise to a viral oncogene (v-onc). It is trig-
gered by proviral integration next to the cellular proto-oncogene creating a viral-cellular fusion gene. RNA processing following read-through tran-
scription removes the introns from this hybrid (viral 1 cellular) RNA sequence. When this hybrid RNA sequence undergoes recombination with the
viral RNA during reverse-transcription, the cellular oncogene is captured by the viral genome without the introns.