Page 653 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 653
618 SECTION | VIII Rodenticides
VetBooks.ir RED SQUILL ingest a product containing scilliroside, because they are
Scilliroside has an emetic property; thus, if rodents
Introduction
incapable of vomiting, they develop glycoside intoxica-
Red squill, which is also known as sea onion, is obtained tion and pulmonary edema. However, it has rarely been
in the powder form from the plant Urginea maritima. The associated with toxicity in humans because humans are
plant is native to the Mediterranean region. It resembles capable of vomiting and exhibit poor GI absorption.
an onion, and its bulb extracts and dried powders have
been used for the control of rodents since the 13th cen- Toxicokinetics
tury. Although red squill has many alkaloids, scilliroside
is the most toxic, and provides rodenticidal activity. It has Scilliroside is inefficiently absorbed from the GI tract. Its
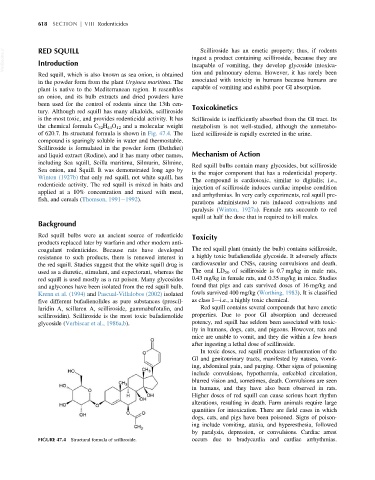
the chemical formula C 32 H 44 O 12 and a molecular weight metabolism is not well-studied, although the unmetabo-
of 620.7. Its structural formula is shown in Fig. 47.4. The lized scilliroside is rapidly excreted in the urine.
compound is sparingly soluble in water and thermostable.
Scilliroside is formulated in the powder form (Dethdiet)
and liquid extract (Rodine), and it has many other names, Mechanism of Action
including Sea squill, Scilla maritima, Silmurin, Silmine,
Red squill bulbs contain many glycosides, but scilliroside
Sea onion, and Squill. It was demonstrated long ago by
is the major component that has a rodenticidal property.
Winton (1927b) that only red squill, not white squill, has
The compound is cardiotoxic, similar to digitalis; i.e.,
rodenticide activity. The red squill is mixed in baits and
injection of scilliroside induces cardiac impulse condition
applied at a 10% concentration and mixed with meat,
and arrhythmias. In very early experiments, red squill pre-
fish, and cereals (Thomson, 1991 1992).
parations administered to rats induced convulsions and
paralysis (Winton, 1927a). Female rats succumb to red
squill at half the dose that is required to kill males.
Background
Red squill bulbs were an ancient source of rodenticide Toxicity
products replaced later by warfarin and other modern anti-
coagulant rodenticides. Because rats have developed The red squill plant (mainly the bulb) contains scilliroside,
resistance to such products, there is renewed interest in a highly toxic bufadienolide glycoside. It adversely affects
the red squill. Studies suggest that the white squill drug is cardiovascular and CNSs, causing convulsions and death.
used as a diuretic, stimulant, and expectorant, whereas the The oral LD 50 of scilliroside is 0.7 mg/kg in male rats,
red squill is used mostly as a rat poison. Many glycosides 0.43 mg/kg in female rats, and 0.35 mg/kg in mice. Studies
and aglycones have been isolated from the red squill bulb. found that pigs and cats survived doses of 16 mg/kg and
Krenn et al. (1994) and Pascual-Villalobos (2002) isolated fowls survived 400 mg/kg (Worthing, 1983). It is classified
five different bufadienolides as pure substances (proscil- as class I—i.e., a highly toxic chemical.
laridin A, scillaren A, scilliroside, gammabufotalin, and Red squill contains several compounds that have emetic
scillirosidin). Scilliroside is the most toxic bufadienolide properties. Due to poor GI absorption and decreased
glycoside (Verbiscar et al., 1986a,b). potency, red squill has seldom been associated with toxic-
ity in humans, dogs, cats, and pigeons. However, rats and
mice are unable to vomit, and they die within a few hours
after ingesting a lethal dose of scilliroside.
In toxic doses, red squill produces inflammation of the
GI and genitourinary tracts, manifested by nausea, vomit-
ing, abdominal pain, and purging. Other signs of poisoning
include convulsions, hypothermia, enfeebled circulation,
blurred vision and, sometimes, death. Convulsions are seen
in humans, and they have also been observed in rats.
Higher doses of red squill can cause serious heart rhythm
alterations, resulting in death. Farm animals require large
quantities for intoxication. There are field cases in which
dogs, cats, and pigs have been poisoned. Signs of poison-
ing include vomiting, ataxia, and hyperesthesia, followed
by paralysis, depression, or convulsions. Cardiac arrest
FIGURE 47.4 Structural formula of scilliroside. occurs due to bradycardia and cardiac arrhythmias.