Page 112 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 112
CHAPTER 4 Epidemiology and the Evidence-Based Medicine Approach 91
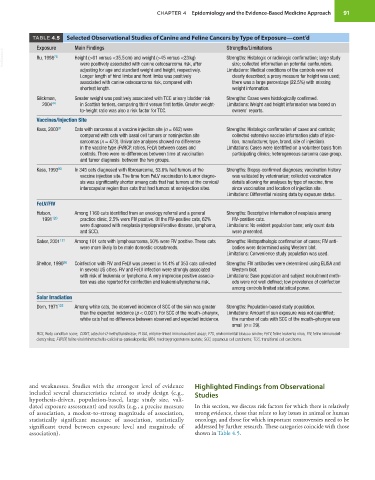
TABLE 4.5 Selected Observational Studies of Canine and Feline Cancers by Type of Exposure—cont’d
Exposure Main Findings Strengths/Limitations
VetBooks.ir Ru, 1998 76 Height (>61 versus <35.5 cm) and weight (>45 versus <23 kg) Strengths: Histologic or radiologic confirmation; large study
size; collected information on potential confounders.
were positively associated with canine osteosarcoma risk, after
adjusting for age and standard weight and height, respectively. Limitations: Medical conditions of the controls were not
Longer length of hind limbs and front limbs was positively clearly described; a proxy measure for height was used;
associated with canine osteosarcoma risk, compared with there was a large percentage (22.5%) with missing
shortest length. weight information.
Glickman, Greater weight was positively associated with TCC urinary bladder risk Strengths: Cases were histologically confirmed.
2004 44 in Scottish terriers, comparing third versus first tertile. Greater weight- Limitations: Weight and height information was based on
to-height ratio was also a risk factor for TCC. owners’ reports.
Vaccines/Injection Site
Kass, 2003 91 Cats with sarcomas at a vaccine injection site (n = 662) were Strengths: Histologic confirmation of cases and controls;
compared with cats with basal cell tumors or noninjection site collected extensive vaccine information (date of injec-
sarcomas (n = 473). Univariate analyses showed no difference tion, manufacturer, type, brand, site of injection).
in the vaccine type (FVRCP, rabies, FeLV) between cases and Limitations: Cases were identified on a volunteer basis from
controls. There were no differences between time at vaccination participating clinics; heterogeneous sarcoma case group.
and tumor diagnosis between the two groups.
Kass, 1993 90 In 345 cats diagnosed with fibrosarcoma, 53.6% had tumors at the Strengths: Biopsy-confirmed diagnoses; vaccination history
vaccine injection site. The time from FeLV vaccination to tumor diagno- was validated by veterinarian; collected vaccination
sis was significantly shorter among cats that had tumors at the cervical/ details allowing for analyses by type of vaccine, time
interscapular region than cats that had tumors at noninjection sites. since vaccination and location of injection site.
Limitations: Differential missing data by exposure status.
FeLV/FIV
Hutson, Among 1160 cats identified from an oncology referral and a general Strengths: Descriptive information of neoplasia among
1991 120 practice clinic, 2.5% were FIV positive. Of the FIV-positive cats, 62% FIV-positive cats.
were diagnosed with neoplasia (myeloproliferative disease, lymphoma, Limitations: No evident population base; only count data
and SCC). were presented.
Gabor, 2001 121 Among 101 cats with lymphosarcoma, 50% were FIV positive. These cats Strengths: Histopathologic confirmation of cases; FIV anti-
were more likely to be male domestic crossbreeds. bodies were determined using Western blot.
Limitations: Convenience study population was used.
Shelton, 1990 96 Coinfection with FIV and FeLV was present in 14.4% of 353 cats collected Strengths: FIV antibodies were determined using ELISA and
in several US cities. FIV and FeLV infection were strongly associated Western blot.
with risk of leukemia or lymphoma. A very imprecise positive associa- Limitations: Base population and subject recruitment meth-
tion was also reported for coinfection and leukemia/lymphoma risk. ods were not well defined; low prevalence of coinfection
among controls limited statistical power.
Solar Irradiation
Dorn, 1971 122 Among white cats, the observed incidence of SCC of the skin was greater Strengths: Population-based study population.
than the expected incidence (p < 0.001). For SCC of the mouth–pharynx, Limitations: Amount of sun exposure was not quantified;
white cats had no difference between observed and expected incidence. the number of cats with SCC of the mouth–pharynx was
small (n = 29).
BCS, Body condition score; COMT, catechol-O-methyltransferase; ELISA, enzyme-linked immunosorbent assay; ETS, environmental tobacco smoke; FeLV, feline leukemia virus; FIV, feline immunodefi-
ciency virus; FVRCP, feline viral rhinotracheitis-calicivirus-panleukopenia; MPA, medroxyprogesterone acetate; SCC, squamous cell carcinoma; TCC, transitional cell carcinoma.
and weaknesses. Studies with the strongest level of evidence Highlighted Findings from Observational
included several characteristics related to study design (e.g., Studies
hypothesis-driven, population-based, large study size, vali-
dated exposure assessment) and results (e.g., a precise measure In this section, we discuss risk factors for which there is relatively
of association, a modest-to-strong magnitude of association, strong evidence, those that relate to key issues in animal or human
statistically significant measure of association, statistically oncology, and those for which important controversies need to be
significant trend between exposure level and magnitude of addressed by further research. These categories coincide with those
association). shown in Table 4.5.